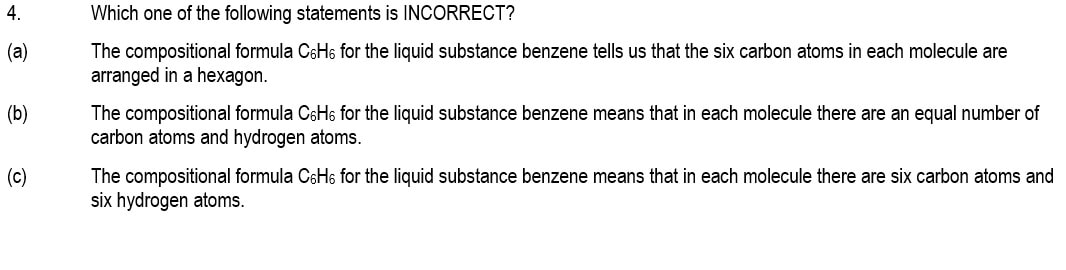Module 0504
Chemical formulas: What can they tell us?
How do we interpret chemical formulas?
That depends .... on?
Things they can tell us, and things they cannot.
What does the formula CO2 tell us?
The formula MgCl2 must be interpreted differently! Why?
Foreshadowing: The answer depends on the type of compound
Interpreting chemical formulas is a very important part of learning chemistry.
All chemical formulas are not interpreted in the same way. But although there is not just one way to interpret chemical formulas, you don't get a choice.
We need to know something about the structure of a substance before we can properly know what its formula tells us.
As you will see in the video ...
Prof Bob and Aussie get deep into what chemical equations can tell us - if you already know something about the stuffs and species.
KEY IDEAS - Interpreting chemical formulas
Firstly, let's get if right .......
Formulas are neither substances nor names
To understand chemistry well, it is important to make the distinction among:
- the substance in question (For example, the colourless liquid that we shower in, or the white crystalline solid that some of us use as a taste enhancer on food)
- its name (Water, sodium chloride)
- its formula (H2O, NaCl).
For example, ethanol is the name of a colourless liquid substance often used in medical situations as a disinfectant. It is not C2H5OH. The formula C2H5OH is a carrier of information about the composition of the molecules of teh substance.
It all depends .......
How the information embedded in a chemical formula is properly interpreted depends upon the category of substance of the compound – in particular whether it is categorised as a covalent molecular compound or an ionic compound.
Interpreting formulas of covalent molecular compounds
Substances that we categorise (on the basis of their properties) as covalent molecular compounds comprise identical molecules (each a group of atoms held together in a certain connectivity by covalent bonds between atoms).
The chemical formula of a covalent molecular compound tells us the actual number of atoms of each element in each of the molecules.
But it tells us nothing about the connectivity of atoms in the molecules (that is, what is bonded to what).
Interpreting formulas of ionic compounds
Substances that we categorise (on the basis of their properties) as ionic compounds, comprise an orderly arrangement of positively charged ions (called cations) and negatively charged ions (called anions). See module 0905 Dissolution of ionic salts in water.
Each cation is surrounded by anions, and each anion by cations. The crystal is held together by the electrostatic forces of attraction between cations and anions (dominating the cation-cation and anion-anion repulsions.
We can think of this ionic bonding as a cooperative effect involving the whole crystal: there are no pairs of ions (or small groups of ions) that can be identified as more-or-less independent units: that is, it is not composed of molecules.
The chemical formula of an ionic compounds can tell us only the relative numbers (the ratio) of cations and anions in the crystal.
And it tells us nothing about the particular orderly arrangement of the cations and anions among each other.
Formulas of molecules vs. formulas of substances
Oh, and another important distinction, not discussed in the above video .....
Contrast, for example:
- The formula C2H5OH refers to the composition of ethanol molecules
- The formula C2H5OH(l) refers to the liquid substance called ethanol.
SELF CHECK: Some thinking tasks

You need to know some stuff before you can properly interpret chemical formulas. Can you see the link between that statement and the snails? Neither can I. Maybe one of them is Prof Bobsnail, and the other is Aussiesnail, and Aussiesnail is learning about interpreting chemical formulas. I hope that he understands well enough to pass the test below ....

6. Two chemistry students walk into a bar. Fatima well understands the importance of chemical formulas, but Dennis hasn't yet watched Prof Bob's video. Fatima says "I'll have an H2O, please". Dennis says "I'll have an H2O too". One of them suffered a painful death. Which one? Why? What is wrong with Fatima's order?
Answers: 1 (c); 2 (a); 3 (c); 4 (a); 5 (d)
6. Dennnis died. He was given a glass of hydrogen peroxide by a barman who knew some chemistry (Think about it ....). What Fatima asked for is the formula showing the composition of water molecules. She actually wanted some of a liquid substance, so she should have asked for some H2O(l).
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.