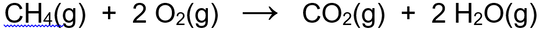Chapter 05 HOME page
Chemical reactions and chemical equations
A chemical reaction is an event.
A chemical equation is a story about the event.
Above, we can see evidence of a chemical reaction occurring. The essence of a chemical reaction is that new chemical species are formed.
And below is a chemical equation: chemists' language that gives us some information about the chemical reaction ....
Vive la difference!
Modules in this Chapter 05 explore the difference between chemical changes (reactions) and their representational form (chemical equations), as well as the significance of both.
Modules in Chapter 05 so far (Click to go):
0500 Chemical reactions vs chemical equations: Overview
0501 Chemical amount and its unit of measurement, mole
0502 The Avogadro constant: How many particles is that?
0503 The Avogadro constant: Why is it that number?
0504 Chemical formulas: What can they tell us?
0505 Chemical equations: And what can't they tell us?
0506 Limiting reactants: How much reaction can happen?
0507 Balanced chemical equations: What are they?
0508 Chemical reactions as competitions
0500 Chemical reactions vs chemical equations: Overview
0501 Chemical amount and its unit of measurement, mole
0502 The Avogadro constant: How many particles is that?
0503 The Avogadro constant: Why is it that number?
0504 Chemical formulas: What can they tell us?
0505 Chemical equations: And what can't they tell us?
0506 Limiting reactants: How much reaction can happen?
0507 Balanced chemical equations: What are they?
0508 Chemical reactions as competitions
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.