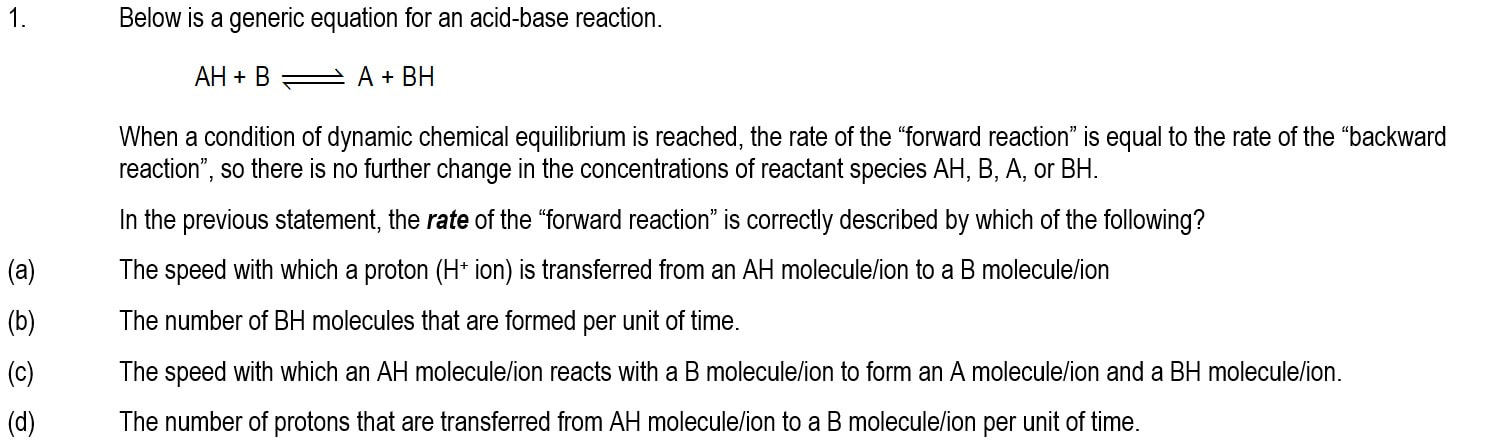
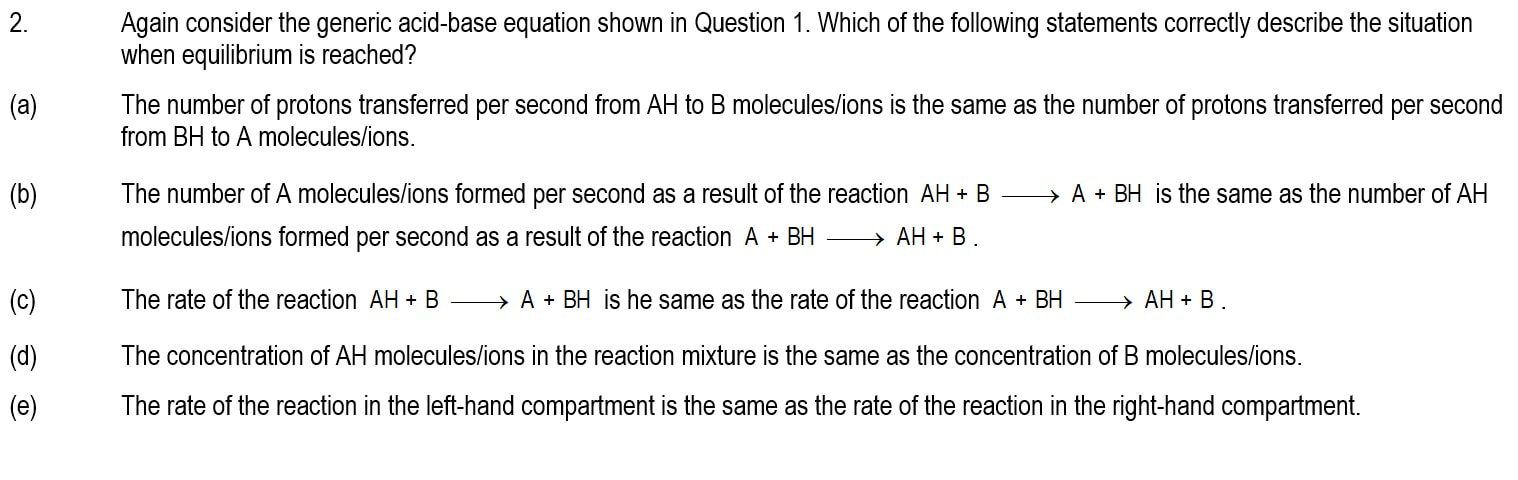
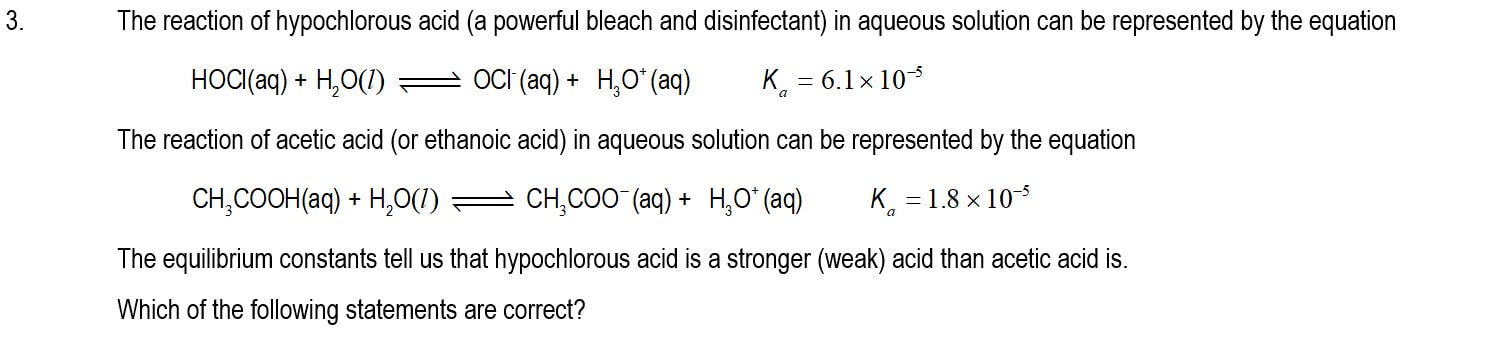
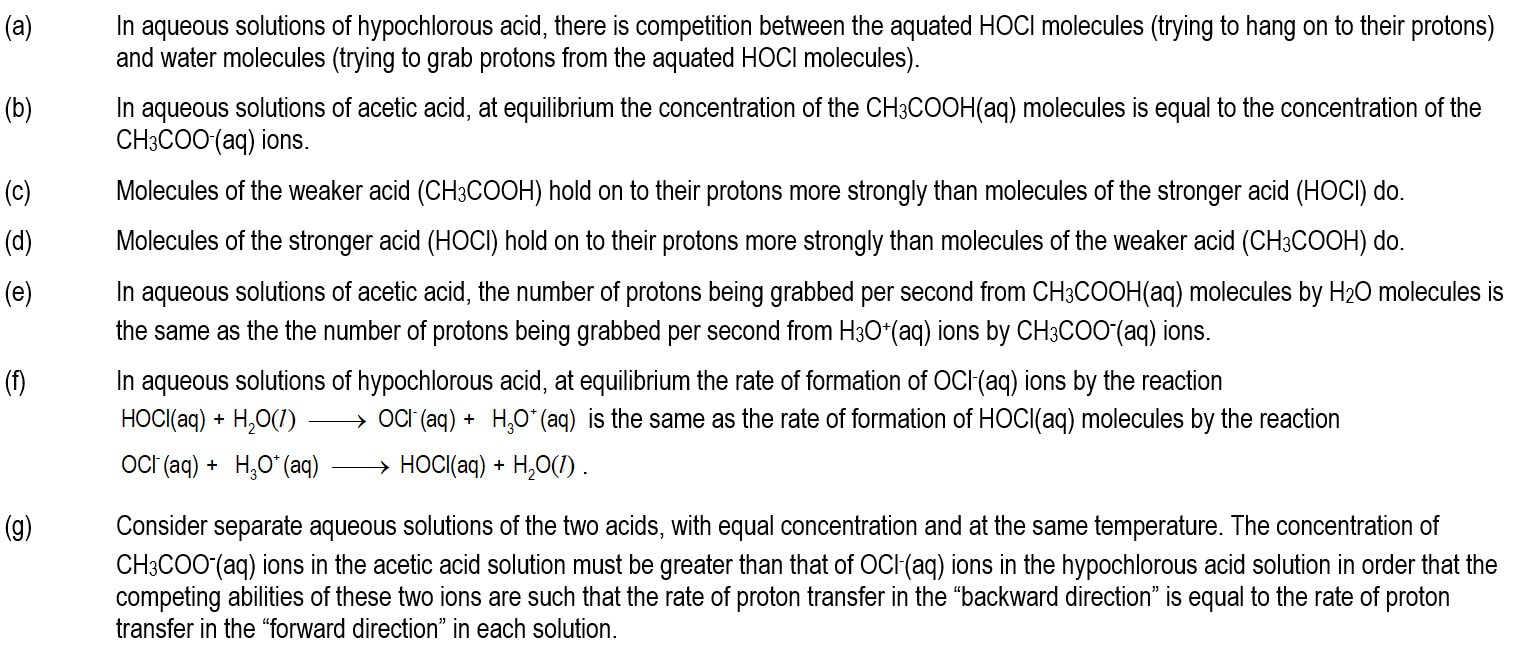
Module 1101
Visualising dynamic chemical equilibrium
What is dynamic about chemical equilibrium?
What does it mean for a reaction to go 'both ways' simultaneously?
Is it a useful mental picture to visualise just one ion/molecule of each of the reaction participants - as you might (wrongly) interpret the equation?
The answer is definitely not! Why not?
Visualise the many molecules of species in the reaction mixture, rather than focus on the reaction equation.
Competition, competition ... always competition. Imagine that you are the species being competed for.
What governs how far reaction goes to reach dynamic chemical equilibrium?
An analogy
What is the condition of dynamic chemical equilibrium? What does it mean that the “forward reaction” and the “backward reaction” occur simultaneously and at the same rate? Prof Bob’s analogy might help to clarify meaning.
Did this analogy help your insight into what is going on in a reaction mixture with trillions upon trillions of ions or molecules of each of the reaction species?
KEY IDEAS - Visualising dynamic chemical equilibrium
Forewarning
Interpreting the language used by chemists is often a problem for those learning chemistry. To a student, the meaning of a phrase or term used by a chemist might be completely misleading. He/she knows what they mean when they use the language of the profession, but until you are familiar with this language, potential trouble looms. This is especially the case in regard to the topic of dynamic chemical equilibrium.
Prof strongly urges you to go to Module 1102 The jargon of chemical equilibrium in conjunction with your sense-making of this module.
You might even find it useful to think about communication in a more general sense by looking at Module 2703 The jargon we use. Are talker and listener on the 'same page'? Is the talker making presumptions about your understanding of words and phrases? Of course, we do that all the time, even in everyday conversations, but it is useful if the presumption is close to correct!
Prof strongly urges you to go to Module 1102 The jargon of chemical equilibrium in conjunction with your sense-making of this module.
You might even find it useful to think about communication in a more general sense by looking at Module 2703 The jargon we use. Are talker and listener on the 'same page'? Is the talker making presumptions about your understanding of words and phrases? Of course, we do that all the time, even in everyday conversations, but it is useful if the presumption is close to correct!
The conditions of chemical equilibrium in a reaction mixture
Chemical reactions as competitive processes
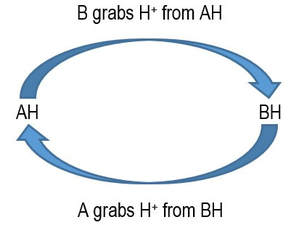
Beware! This representation might suggest that chemical equilibrium is the result of one A and one B competing for a single H+ ion. No, no, no! That might be the case in a single collision between AH and B (or between BH and A), but there are trillions upon trillions of such collisions happening all the time. To visualise a reaction mixture, you need to try to picture the overall result arising from these many many collisions happening every moment.
Changing relative amounts of 'reactants' and 'products'
Visualise yourself as the object of competition
NOTE TO TEACHERS
For some thoughts about pedagogical content knowledge related to this concept, see Teachers' Corner page
PCK1101: Visualizing dynamic chemical equilibrium.
For some thoughts about pedagogical content knowledge related to this concept, see Teachers' Corner page
PCK1101: Visualizing dynamic chemical equilibrium.
SELF CHECK - Some thinking tasks
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.
Seek wisdom, insight and understanding. Satisfaction will follow.