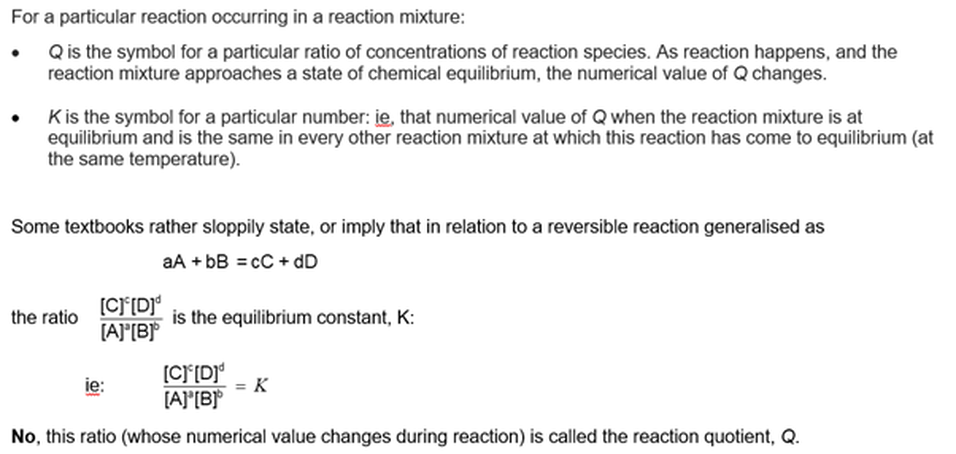
Module 2203
Ultraviolet-visible spectroscopy
Why do solutions of different substances have different colours?
Why are none of the colours identical?
Why are different wavelengths of white light absorbed?
What is quantisation of energy?
Why do solutions not absorb some wavelengths?
Prof Bob takes the general ideas discussed in Module 2201 to a more specific level .....
Prof Bob does classroom demonstrations of visible spectroscopy in the classroom and in the garden. Is the pertinent question why some wavelengths of radiation are NOT absorbed by species in solution?
KEY IDEAS - UV-visible spectrosocopy
In Module 2201, Prof Bob talks in general terms about spectra across the whole of the electromagnetic spectrum: elephants to radio waves. Here he focusses on spectra in the ultraviolet and visible range of wavelengths ..... well, he only demonstrates visible spectra actually, but the origins of spectra across the visible and ultraviolet regions are the same.
A level of explanation: Which wavelengths are absorbed?
Which are transmitted?
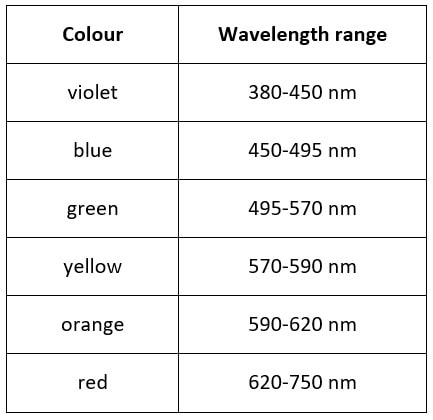
At a simple level of explanation, a solution that appears red to us absorbs, from the white light striking it, blue and green light - so that only “red wavelengths” are transmitted. Yellow solutions absorb blue light, and blue solutions absorb red/yellow/orange light from white light.
[I have used the term “red wavelengths” in quotation marks because wavelengths cannot be coloured. This is a shorthand way of referring to wavelengths of radiation which we perceive by eye as red colour.]
This leads to the question of why a solution absorbs only particular wavelengths of light (or photons with particular energy). And to the equally important question of why the solution does not absorb all of the other wavelengths from white light.
Why are only particular wavelengths absorbed?
And the others not?
The scientists’ explanation is quantization of the energy levels of each mode ….
All modes of energy of molecules, ions and atoms (electron energy, vibrational energy, rotational energy) are quantized: that is, the energy of each mode can have only particular levels – and cannot have energy intermediate between those levels.
Pause for some terminology .......
- An "allowed" energy level means an energy level that is possible (because that is the way things are, and not in the sense of being allowed by persons, or by the law). Allowed by Mother Nature? A chemical species cannot exist at an energy level that is not “allowed”.
- "Excitation" refers to the increase of energy to a higher “allowed” level – in this case, of electrons, but the term is also applicable to vibrational and rotational energy levels.
“Excitation” of any energy mode can only happen as a result of absorption of photons whose energy corresponds exactly with the energy gap between any two “allowed” energy levels. Otherwise, the energy would reach a level that is not “allowed”.
The key: The energy gaps between levels of energy of electrons in atoms, molecules or ions (and not the energy levels of vibration or rotation) just happen to be (Mother Nature again!) such that the photons absorbed are those of visible, or near-ultraviolet, radiations.
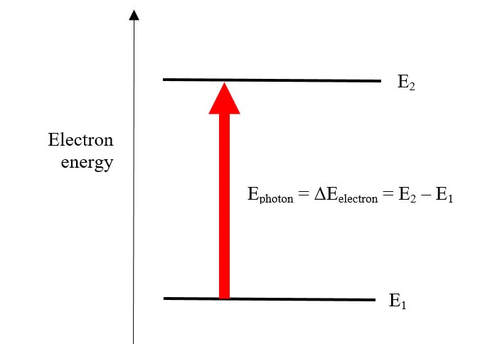
From incident radiation with visible and near-ultraviolet wavelengths, 'excitation' of electrons is only brought about by photons whose energies exactly match the difference of energy between any two 'allowed' energy levels of the electrons - portrayed here for levels E1 and E2, although there are many more 'allowed' levels. Otherwise, the electron would be 'excited' to an energy level that is not 'allowed'.
Of course different species have different “allowed” energy levels, and different energy gaps. So the photons absorbed are of different energies – and so the light transmitted (that is, not absorbed) is different for different species.
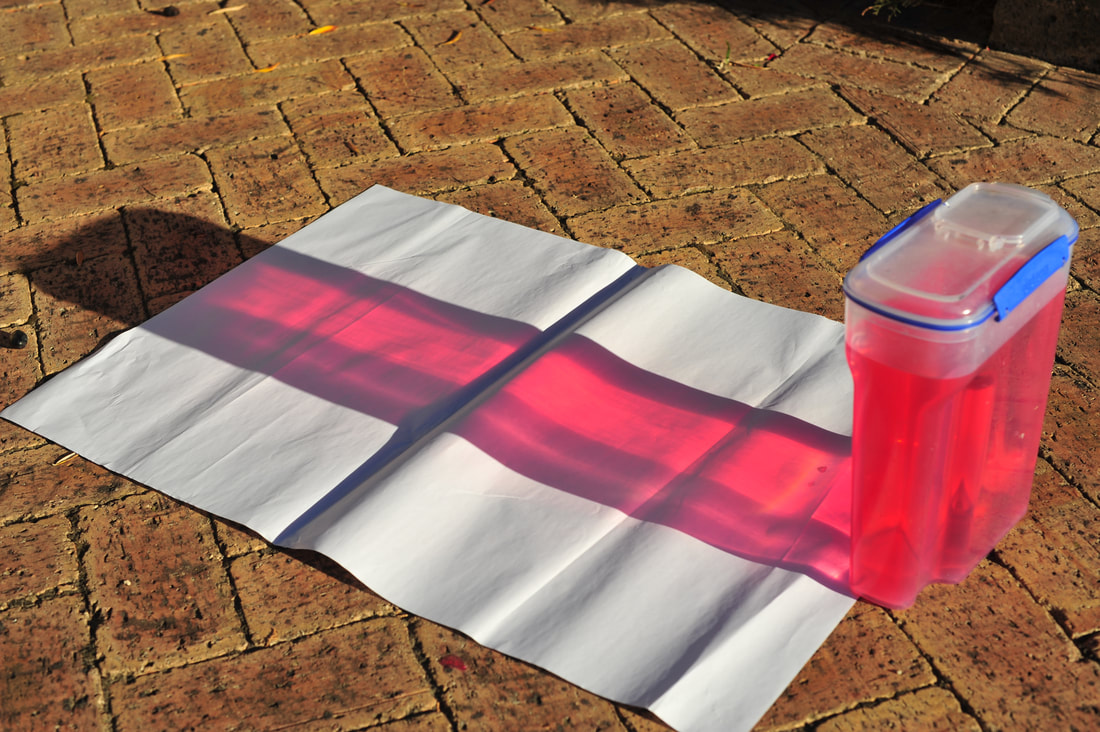
Coloured partial shadows
If an opaque object is placed in the path of white light, a shadow is produced. This is total prevention of any of the white light from passing through.
We can physically form a partial shadow if the white light passes through a mesh. Some fraction of the white light is prevented from passing throught to a surface. But that which does pass through is also white light - all of the wavelengths of visible light.
We can form coloured partial shadows by chemical means. For example, a blue solution put in the path of white light gives rise to a blue partial shadow because some components of the white light (red/orange light) are absorbed and do not pass through.
And a red partial shadow is formed as a result of ......... ?
Thinking, thinking ......... What is the colour of a shadow formed when white light passes through, in succession, a blue solution, a yellow solution, and a red solution? See Prof Bob's video at the10-minute mark.
A clarification
Red light (for example) can be the result of two different spectroscopic processes:
Red light (for example) can be the result of two different spectroscopic processes:
- Some substances can emit red light when the energy of their electrons fall to a lower “allowed” energy. A plot of the intensities of wavelengths emitted is an emission spectrum (See Module 2201: Quantization of forms of energy).
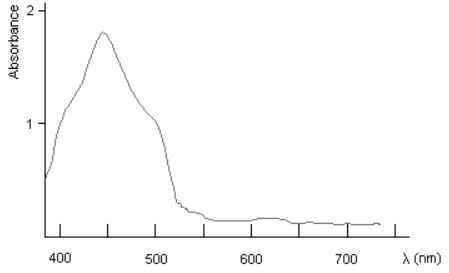
- And some can transmit red light when blue/green light is absorbed as the energy of their electrons increases to a higher “allowed” energy. A plot of the fractions of wavelengths absorbed is an absorption spectrum.
SELF CHECK - Some thinking tasks
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.