Is climate change real?
If it is, what contribution to change has been brought about by human activities?
What is the "greenhouse effect"?
And the "enhanced greenhouse effect"?
Which substances are "greenhouse gases"? Why?
Is carbon dioxide in our atmosphere good or bad?
Another term used for the phenomenon discussed in this chapter is global warming.
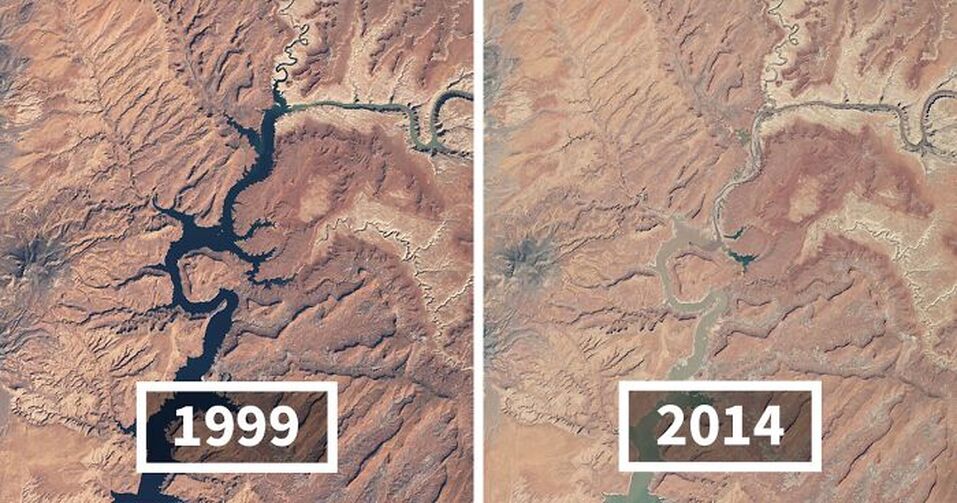
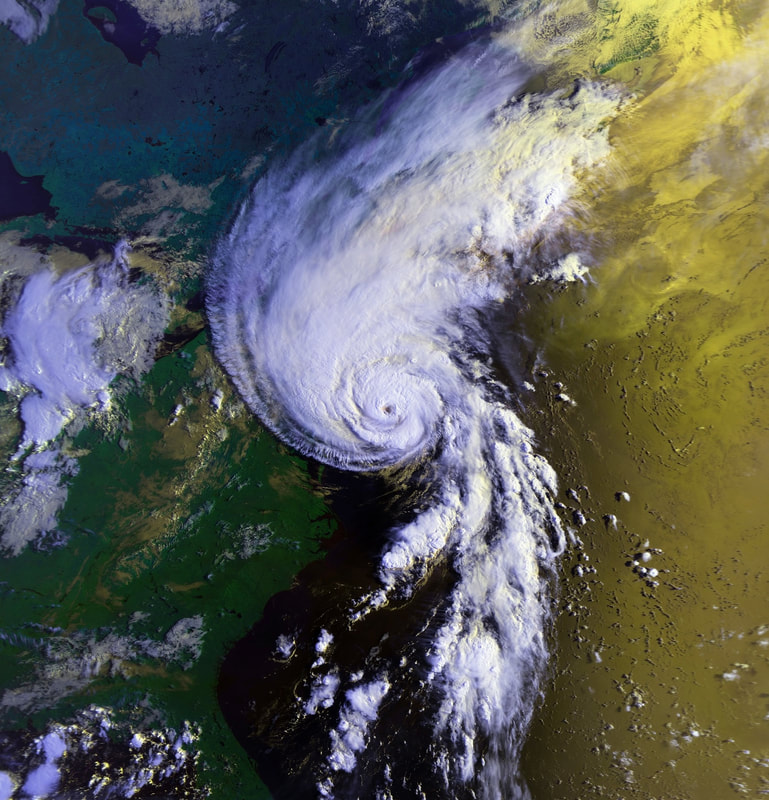
On its own, none of the recent photos below can prove that climate change is real - but the total story is convincing, especially in conjunction with the measurements that reputable scientific communities are making.
Credit: NASA
Let the science lead discussions ....
Almost all scientists accept that climate change is real and a danger to humanity - as do I - because the science makes sense, and the data is undeniable. But when was the last time that you heard our decision-makers discussing the science of climate change? The science has been buried under politics, economics and short-term self-interest.On this site, I will not be discussing political (non-)responses to the threat, nor the serious economic and social consequences, nor ways to limit climate change. Just the underlying science of the phenomenon.
The science of climate change through questions
With regard to climate change, the following are some questions posed by curious people, doubters, deniers, and those who have a self interest in the fossil fuel industries. Sometimes the questions are asked out of curiosity, and sometimes they are intended to mislead or confuse people. Sensible responses depend on a knowledge of the basics of the relevant science - mainly chemistry and physics.
Many people, including our decision-makers at the highest levels, know only that “carbon dioxide ‘captures’ heat”. What does that mean? And so what?
In this chapter we will address, module by module, the science underlying some of the commonly asked questions. Click to go directly to the module (if the question is in bold and blue), or use the LEARNING MODULES tab at the top of the pages.
- What is meant by the “energy balance” of Earth? Is Earth in energy balance?
- Was carbon dioxide present in the atmosphere before the industrial revolution?
- Since the concentration of carbon dioxide is so small, can it significantly affect what happens in the atmosphere?
- Does carbon dioxide in the atmosphere affect the energy balance of Earth?
- How does carbon dioxide in the atmosphere affect the energy balance of Earth? What is the greenhouse effect” What are “greenhouse gases”?
- How/Why do carbon dioxide molecules “trap” the radiation which is emitted from the surface of Earth?
- How much has the concentration of carbon dioxide increased since 1800. So what?
- Carbon dioxide molecules absorb outgoing radiation from Earth. Why, then, don’t they absorb the incoming radiation from the sun?
- Nitrogen and oxygen are not greenhouse gases. Why do carbon dioxide molecules absorb outgoing radiation from Earth, but nitrogen and oxygen molecules do not?
- Water vapour is the most important greenhouse gas It absorbs 60% of outgoing infrared radiation. The concentration of water vapour in the atmosphere (average 4%) is many times higher than the concentration of carbon dioxide. So, how can an increase in the small concentration of carbon dioxide significantly increase absorption of the outgoing radiation?
- What is the source of carbon dioxide from our vehicles? How is it formed? How much is formed?
- Combustion of petrol produces heat. Where does the energy come from?
- Petrol, coal and natural gas are fuels. How are they different in terms of (i) the amount of carbon dioxide produced, (ii) energy produced, and (iii) “dirty” products of combustion?
- Methane is also a “greenhouse gas”. How does it compare with carbon dioxide?
- What relationships are there between these environmental problems , all of which occur in the atmosphere: (i) the “greenhouse effect”, (ii) depletion of the ozone layer, (iii) acid rain, and (iv) photochemical smog?
- How does an increase in concentration of carbon dioxide in the atmosphere lead to “acidification” of sea water? In this context, what does “acidification” mean? So what?
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.