Module 1103
Equilibrium constants: The law of equilibrium
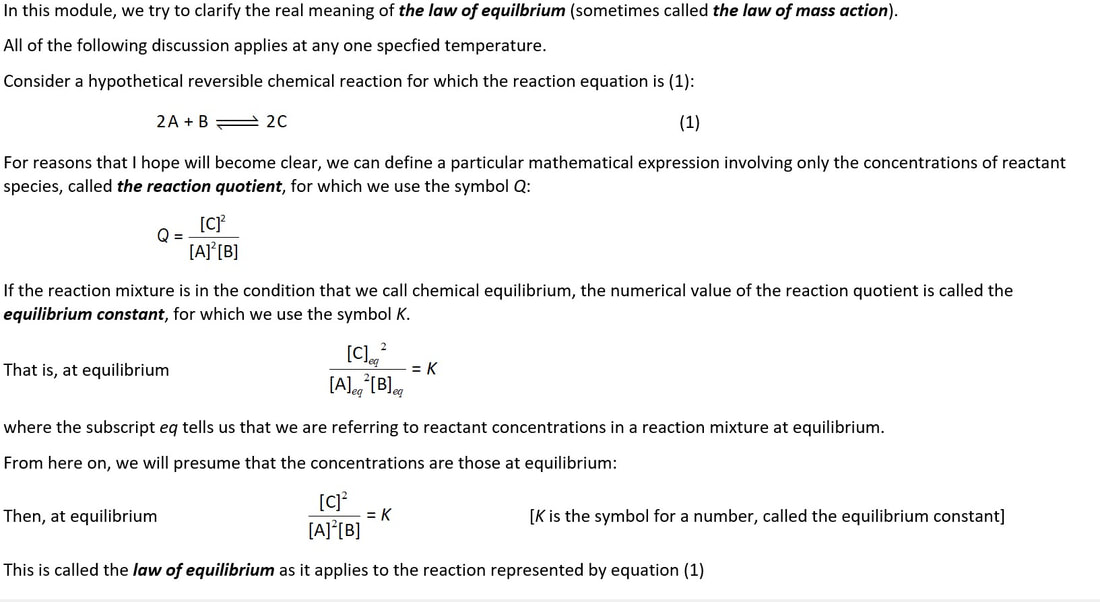
What is the deep meaning of an equilibrium constant, K?
What is the distinction between Q and K?
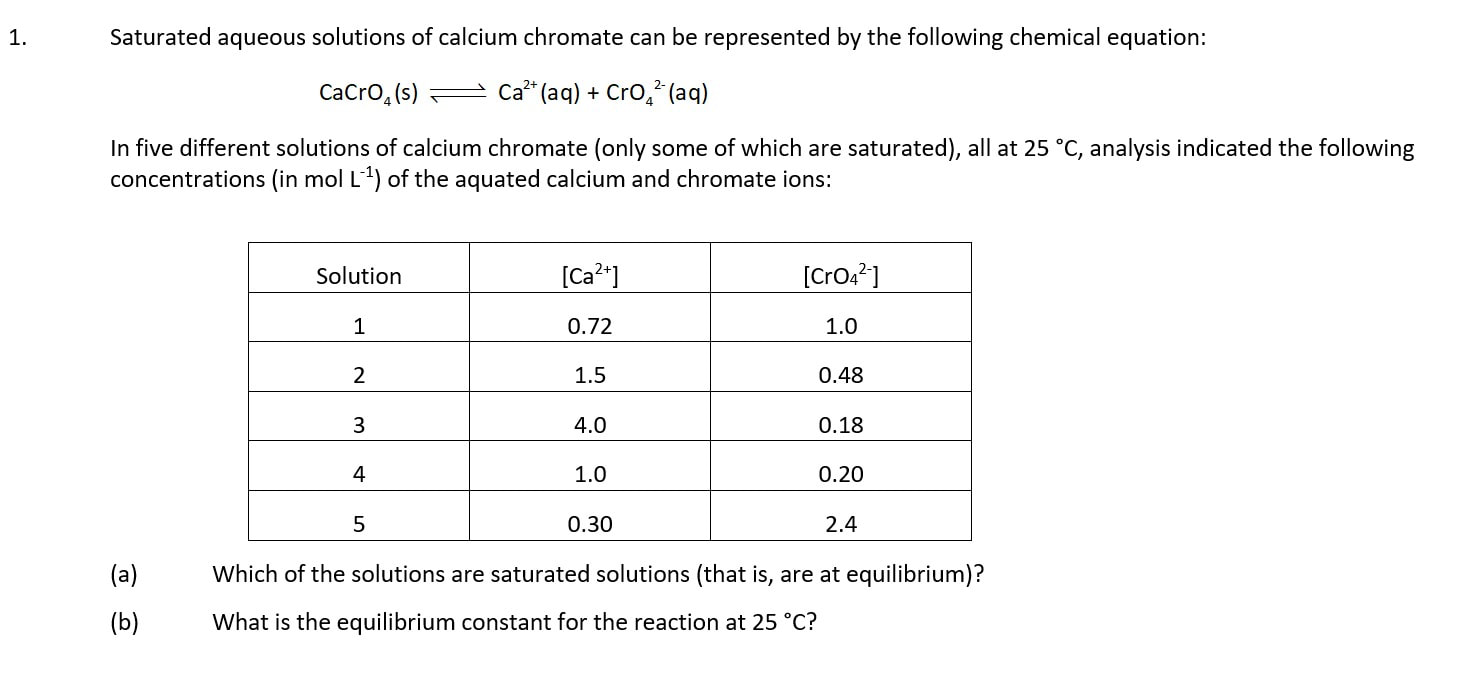
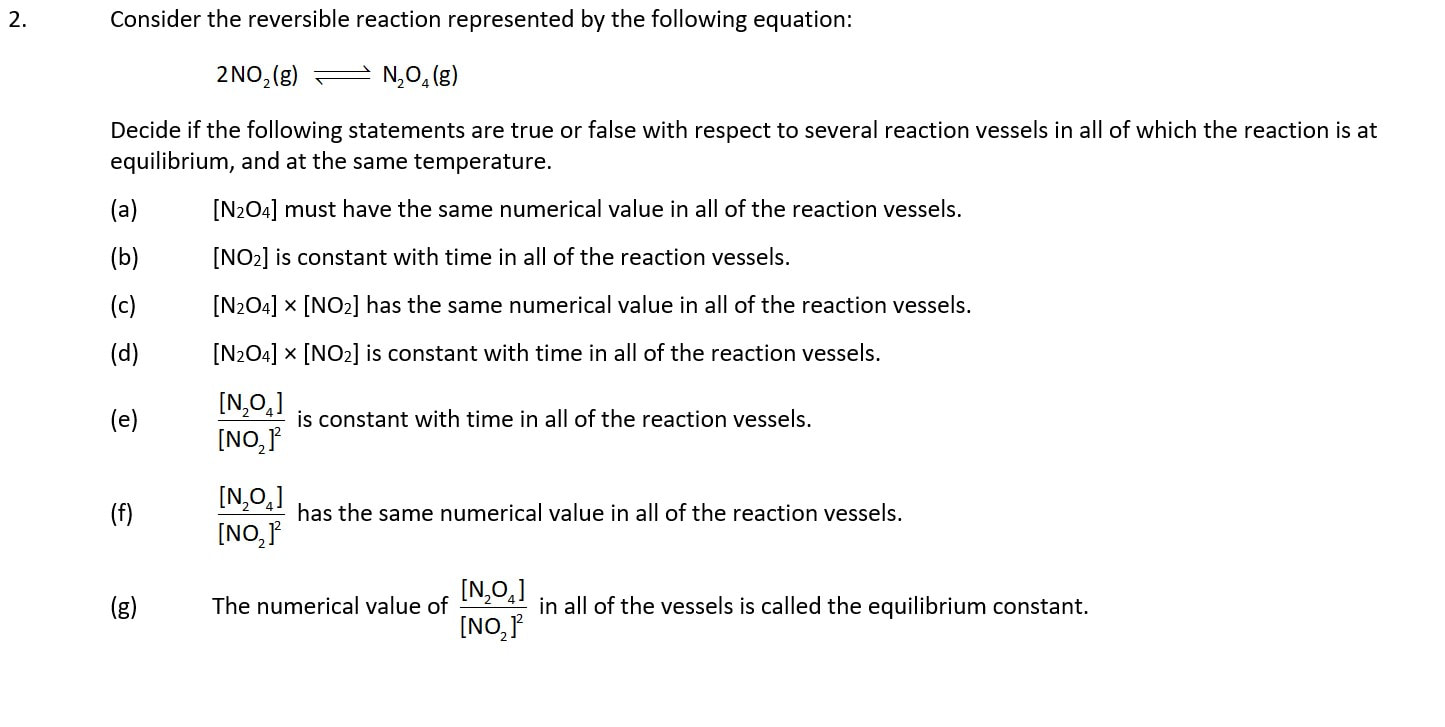
We need to think about more than one reaction mixture to understand the significance of K
Which is the magic word ...... 'constant' or 'the same'?
Prof Bob believes that equilibrium constants have a much more important (and powerful) meaning than often stated or implied common definitions. I don't like to begin in a negative way, but here are two definitions of equilibrium constant that are common 'out there'. You can can compare with other definitions in textbooks and on the internet ......
- The equilibrium constant, K, expresses the relationship between products and reactants of a reaction at equilibrium in a reaction mixture.
- The equilibrium constant is the ratio of the equilibrium concentrations of the products raised to the power of their stoichiometric coefficients to the equilibrium concentrations of the reactants raised to the power of their stoichiometric coefficients
Oh no! So, so much more significant than that! Let me try to explain, with Elena's help .......
Elena has some strong Aha! moments as she comes to comprehend the deeper meaning of equilibrium constants.
KEY IDEAS - Equilibrium constants: The law of equilibrium
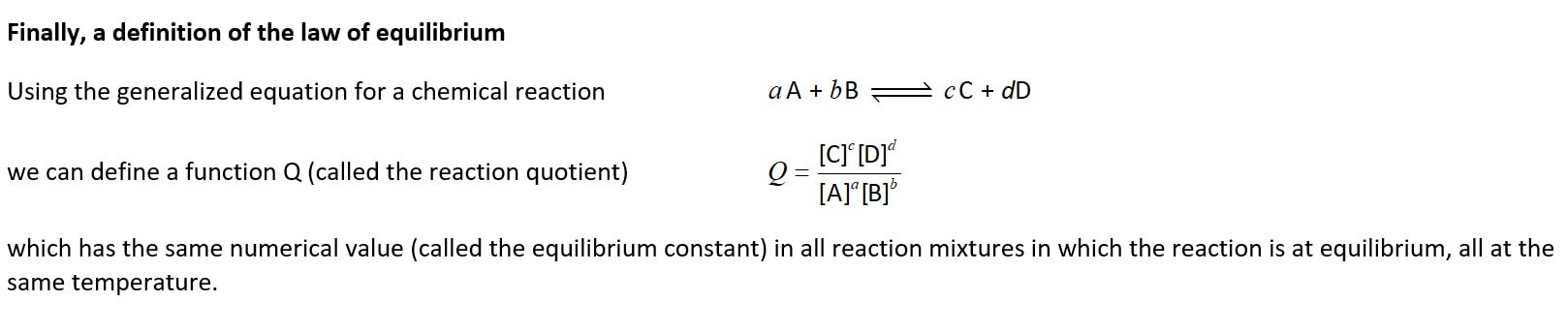
I hope you notice that in this definition the word 'constant' (implying staying at a fixed value over time) is not used. Rather the definition uses 'the same' (in reference to numerical value of Q in all mixtures in which a particular reaction is at equilibrium) if they are at the same temperature.
Chemists' language again ......... To scientists, the word 'constant' means 'the same' in all specified systems. But in everyday usage, the word 'constant' means not changing over time.
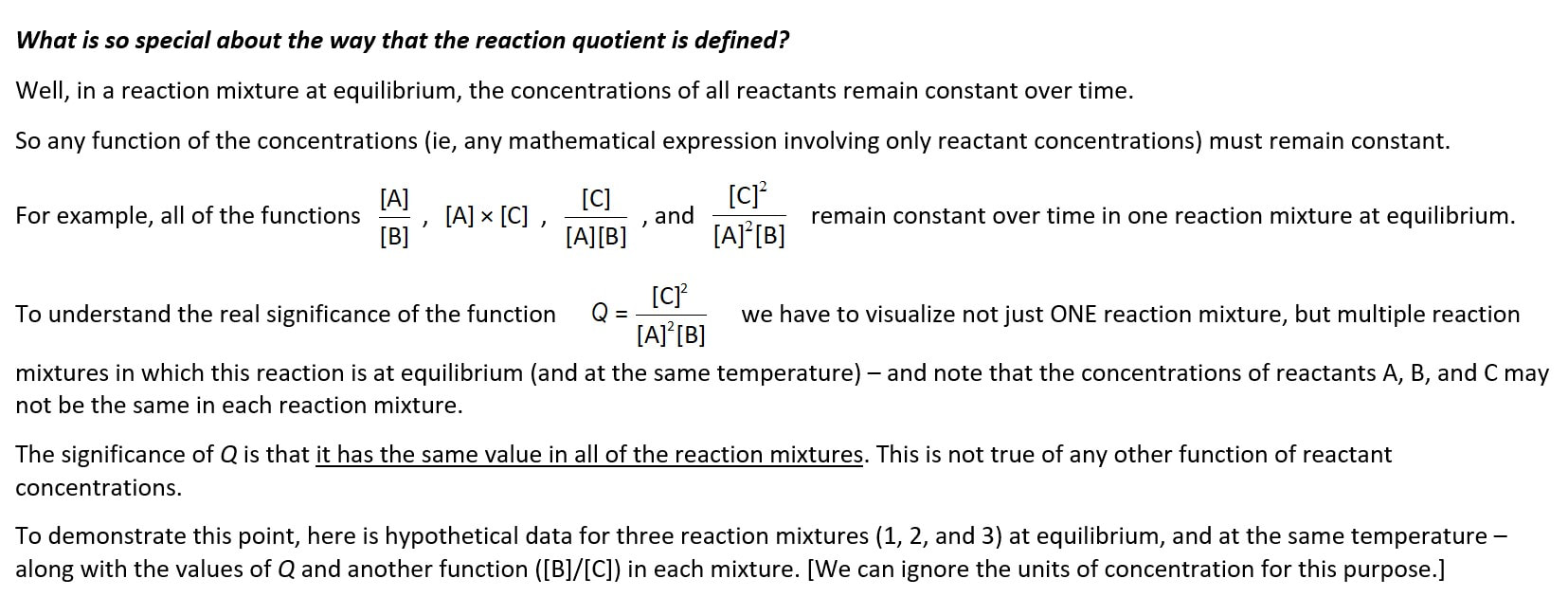
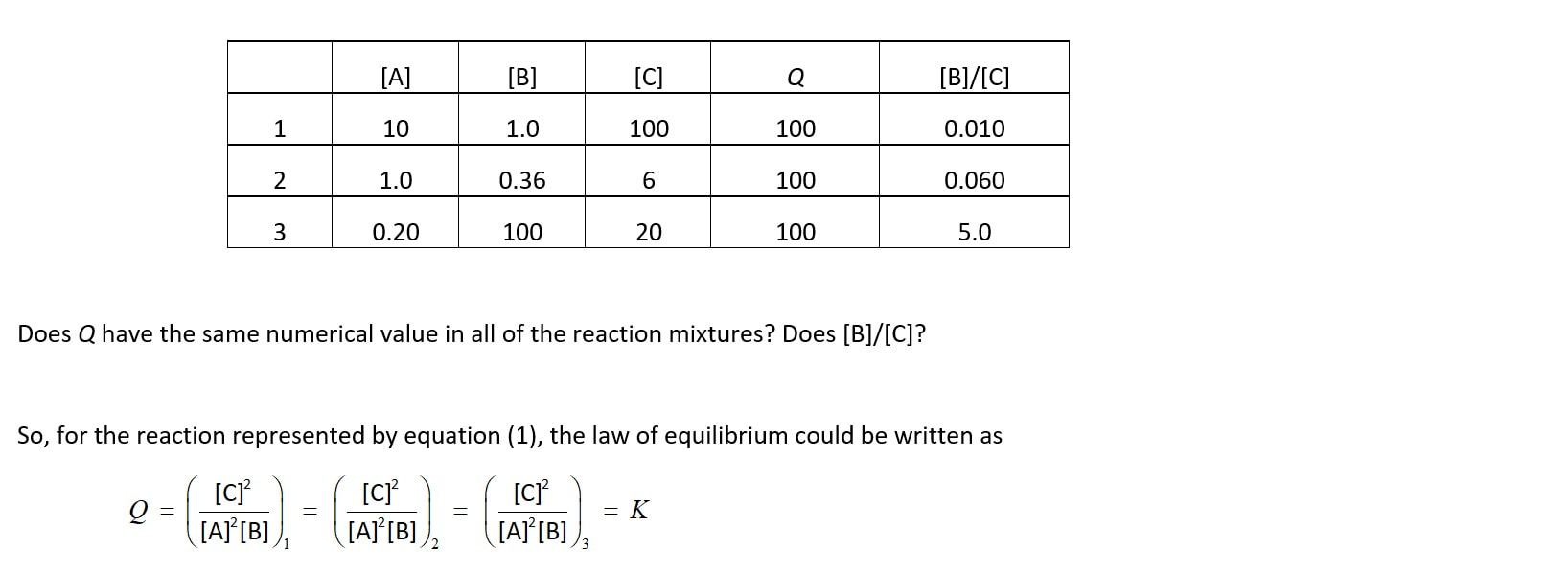
Of course, the numerical value of Q in a single reaction mixture at equilibrium does remain constant over time, but so does any other expression involving only the concentrations of the reaction species - so this idea of constancy is only a small part of the meaning and power of equilibrium constants, and the law of equilibrium.
Chemists' language again ......... To scientists, the word 'constant' means 'the same' in all specified systems. But in everyday usage, the word 'constant' means not changing over time.
Of course, the numerical value of Q in a single reaction mixture at equilibrium does remain constant over time, but so does any other expression involving only the concentrations of the reaction species - so this idea of constancy is only a small part of the meaning and power of equilibrium constants, and the law of equilibrium.
Teachers' note: Pedagogical content knowledge
For a discussion of PCK specifically related to the content of this module, go to Teachers' Corner page PCK1103 Equilibrium constants: The law of equilibrium.
For a discussion of PCK specifically related to the content of this module, go to Teachers' Corner page PCK1103 Equilibrium constants: The law of equilibrium.
SELF CHECK - Some thinking tasks
4. What is wrong with the definitions of equilibrium constant expressed at the start of this module?
4. The statements are correct, but grossly inadequate in terms of the significance of the law of equilibrium in that they do not refer to the equality of values of K in different reaction mixtures at equilibrium (same reaction, same temperature).
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.
Knowledge is good. Knowledge plus wisdom is wonderful! [So they say ...]