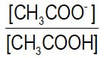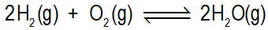Module 1102
The jargon of chemical equilibrium
Reversible reactions?
Direction of reaction?
Does it makes sense to talk about reactants and products?
Net reaction? Net rate of reaction?
Position of equilibrium? Shifts to the left?
Spontaneous?
Every profession has its peculiar terms and phrases - as does chemistry. In relation to the topic of dynamic chemical equilibrium, chemists use language that cannot be interpreted without 'insider information'. A non-chemist might think it to be the language of Martians. No, it's worse than that! You will find, when listening to Martians, that you have no idea what they and saying. But if you listen to chemists, you will often think that you understand, but perhaps entirely mis-understand their meaning, and develop wrong conceptions. But the chemists know what they mean! Sometimes those learning chemistry learn to parrot the terms and phrases for test purposes - without understanding what they really mean.
Over to Prof Bob .....
Let's hope that Prof Bob's clarification of the meaning of terms helps you to understand the concepts of dynamic chemical equilibrium that you will encounter in future .....
KEY IDEAS - the jargon of chemists
In general, about communication and jargon ....
Communication is about transfer of meaning
In an educational context, communication is not just one way. Even in a rather didactic situation, when a teacher is transferring meaning by instruction, and questioning to check learning, every student response to a question is an attempt to transfer meaning to the teacher.
Communication failure is not just the result of one party’s lack of understanding of the other: there is also an issue of mis-understanding: that is, forming a meaning different from that of the speaker/writer.
One of the contributing factors to mis-understanding can be lack of, or different understanding, of the meaning of the language used. Language specific to a particular topic is what we call the jargon of that topic. Just as there is a jargon of football, and of criminal law (for example), so there is a jargon used in discussion of dynamic chemical equilibrium in chemistry.
Discussions about reversible reaction, and systems in a condition of dynamic chemical equilibrium, are full of specific jargon. Even the label that we use (dynamic chemical equilibrium) is jargon-loaded. In relation to this topic the term equilibrium means something different from its use in relation to human emotions, or physical systems in balance.
The jargon peculiar to this topic includes the terms reactants and products, rate of reaction, net reaction, net reaction rate, perturbation of a system at equilibrium, equilibrium shift to left/right, and spontaneity.
If there is not a reasonable degree of commonality of what these terms mean, mis-communication is probable.
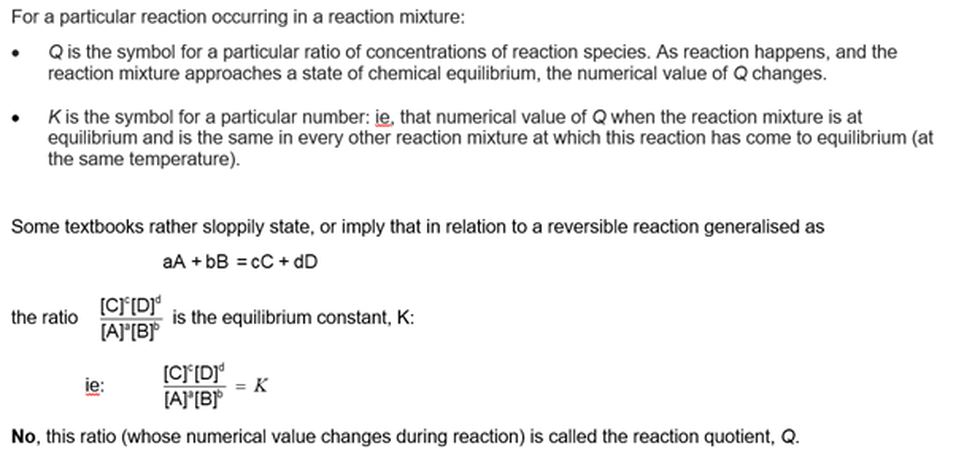
We will ignore here the important concepts of reaction quotient (Q) and equilibrium constant (K) because they are defined and discussed in some detail in Module 1103 Equilibrium constants: The law of equilibrium .
Chemists' jargon - dynamic chemical equilibrium
Let’s use the following chemical equation usually used to represent ionization of acetic acid (ethanoic acid) to illustrate some ideas:
We will see that for precise meaning of terms, it is important to distinguish between the reaction and its representation as a chemical equation. Try to get into the habit of visualizing the molecules and ions in the solution (reaction mixture), rather than the equation.
Now to some specific terms used by chemists in this topic .....
Reversible reactions
If the species in the equation are present in a reaction mixture, then both of the changes
and
are happening simultaneously (at the same time). Each of these is the reverse (the opposite) of the other.
Reversible reactions are represented by the double arrow symbol:
Reversible reactions are represented by the double arrow symbol:
This symbol does not mean that the reaction is at equilibrium – it may be, or maybe not.
Such reactions are capable of achieving a condition of dynamic chemical equilibrium.
Such reactions are capable of achieving a condition of dynamic chemical equilibrium.
Direction of reaction
In the case of a reversible reaction like the one represented above, it is common language to say that one of the reactions goes in the “reverse direction” (or “opposite direction”) to the other. If we were talking about cars on the highway, this would involve changes of location of both cars.
But in the context of a reaction mixture, the only directionality of location is in the way the equations are written – and has no meaning in the sense of change of position of the species. Chemical reactions don't have direction in the sense of a change of location.
The arrow in a chemical equation like ...
But in the context of a reaction mixture, the only directionality of location is in the way the equations are written – and has no meaning in the sense of change of position of the species. Chemical reactions don't have direction in the sense of a change of location.
The arrow in a chemical equation like ...
does not mean "moves to". Rather, it means "changes into".
Let's imagine that we can zoom in to a tiny, tiny region of an acetic acid solution. The equation for the reversible reaction going on is, as before
Let's imagine that we can zoom in to a tiny, tiny region of an acetic acid solution. The equation for the reversible reaction going on is, as before
Every instant, trillions of collisions are happening between species participating in the reaction.
At any instant we might be able to identify collisions that give rise to chemical reaction, pictorially represented here:
At any instant we might be able to identify collisions that give rise to chemical reaction, pictorially represented here:
At any one instant, in many places in the reaction mixture, there are:
There is no change of location of the species in a particular direction.
Summarising, reaction in one 'direction' refers to change of acetic acid molecules to aquated acetate ions. Reaction in the opposite 'direction' is the transformation of acetate ions into acetic acid molecules - the opposite direction of the chemical equation.
- some collisions (let's say, the red) ones) as a result of which a hydrogen ion is transferred from an acetic acid molecule to a water molecule, and
- some collisions (blue), which result in a hydrogen ion being transferred from a hydronium ion to an acetate ion.
There is no change of location of the species in a particular direction.
Summarising, reaction in one 'direction' refers to change of acetic acid molecules to aquated acetate ions. Reaction in the opposite 'direction' is the transformation of acetate ions into acetic acid molecules - the opposite direction of the chemical equation.
'Reactants' and 'products'
First, consider, for comparison, the burning of methane in air, represented by the following equation:
This reaction is essentially “complete” : it is not a reversible reaction, and the reaction proceeds with formation of the carbon dioxide and water vapour until there is no more of either methane or oxygen left in the reaction vessel.
In this case, there is no doubt that the carbon dioxide and oxygen gases are the reactants (they are what react) and carbon dioxide and water vapour are the products (they are what is produced).
However, such labelling is ambiguous in the case of a reaction mixture in which the following reversible reaction is happening:
In this case, there is no doubt that the carbon dioxide and oxygen gases are the reactants (they are what react) and carbon dioxide and water vapour are the products (they are what is produced).
However, such labelling is ambiguous in the case of a reaction mixture in which the following reversible reaction is happening:
Such a reaction mixture might result from various starting situations, such as the following:
In these cases, it is not so clear that we can define what are the reactants, and what are the products.
Really, all of the species are reactants - they all take part in the reaction. So, all of them can be referred to as 'reaction species'.
We should bear this in mind when we engage in the common practice of using the term reactants for the species written on the left-hand side of the equation, and the term products for those written on the right-hand side.
- Adding acetic acid to water.
- Adding a solution of an acid (containing hydronium ions) to a solution of sodium acetate (containing acetate ions).
- Mixing an acetic acid solution, a sodium acetate solution, and a solution of an acid.
- Adding a solution of an acid with an acetic acid solution.
In these cases, it is not so clear that we can define what are the reactants, and what are the products.
Really, all of the species are reactants - they all take part in the reaction. So, all of them can be referred to as 'reaction species'.
We should bear this in mind when we engage in the common practice of using the term reactants for the species written on the left-hand side of the equation, and the term products for those written on the right-hand side.
Net reaction
If a reversible reaction is at equilibrium, the concentrations of all species remain constant. But we cannot say that there is no reaction – there is reaction both ways. But there is no net reaction: no change in the amounts of species present.
If a reaction mixture is not at equilibrium, there will be reaction in one way faster than the other. The amount of any one species will increase as a result of one of the reactions, and decrease by the other. The change in amount of it over a given time period (increase or decrease) is the net reaction.
An over-simple analogy: If we put 100 marbles into a box with one hand, while we take out 75 with the other hand, there is a net change (increase) of 25 marbles.
If, in a given period of time, more of the “reaction to the right” happens than the “reaction to the left”, then there will have been a net increase of species written on the right of the equation.
We say that there was net reaction “to the right”. [I try to avoid that terminology: I would rather say that there has been net reaction to form more acetate ions.
Or, we represent the net reaction by an equation with a single arrow, such as ...
If a reaction mixture is not at equilibrium, there will be reaction in one way faster than the other. The amount of any one species will increase as a result of one of the reactions, and decrease by the other. The change in amount of it over a given time period (increase or decrease) is the net reaction.
An over-simple analogy: If we put 100 marbles into a box with one hand, while we take out 75 with the other hand, there is a net change (increase) of 25 marbles.
If, in a given period of time, more of the “reaction to the right” happens than the “reaction to the left”, then there will have been a net increase of species written on the right of the equation.
We say that there was net reaction “to the right”. [I try to avoid that terminology: I would rather say that there has been net reaction to form more acetate ions.
Or, we represent the net reaction by an equation with a single arrow, such as ...
And here is how we might visualize what "net reaction to the right" means:
At any instant, there are more collisions (red) producing acetate ions than there are collisions (blue) producing acetic acid molecules.
Net rate of reaction
Rate of reaction is the change in the amount (or concentration) of a species in a unit of time. In a reaction mixture not at equilibrium, the net rate of reaction is the difference between the rates of the opposite reactions.
By analogy …
Imagine an all-day concert open from from 9 a.m. until 5 p.m. People can go whenever they like, for as long as they like. Here are some possible scenarios about the rates at which the number of people in the concert changes.
By analogy …
Imagine an all-day concert open from from 9 a.m. until 5 p.m. People can go whenever they like, for as long as they like. Here are some possible scenarios about the rates at which the number of people in the concert changes.
At 10 am: Rate of arrival: 2000 people per hour
Rate of departure: 300 people per hour
Then we say that the net rate of change is an increase of 1700 people per hour.
At 4 pm: Rate of arrival: 450 people per hour
Rate of departure: 1160 people per hour
The net rate of change is a decrease of 710 people per hour.
[In a reaction mixture at equilibrium, the net rate of reaction (net rate of change of amounts of species) is zero.]
Rate of departure: 300 people per hour
Then we say that the net rate of change is an increase of 1700 people per hour.
At 4 pm: Rate of arrival: 450 people per hour
Rate of departure: 1160 people per hour
The net rate of change is a decrease of 710 people per hour.
[In a reaction mixture at equilibrium, the net rate of reaction (net rate of change of amounts of species) is zero.]
Perturbing/Disturbing reaction mixtures at equilibrium
Imagine a reaction mixture in which a particular reaction is at equilibrium. It is possible to change the conditions so that the reaction is (at least instantaneously) no longer at equilibrium. We say that the condition of equilibrium has been perturbed, or disturbed.
This can be done by either (i) changing concentrations of one or more of the species, or (ii) changing the temperature.
After perturbation of the equilibrium, there will be net reaction in the direction that brings the reaction mixture again to a (usually different) condition of equilibrium.
This can be done by either (i) changing concentrations of one or more of the species, or (ii) changing the temperature.
After perturbation of the equilibrium, there will be net reaction in the direction that brings the reaction mixture again to a (usually different) condition of equilibrium.
Position of equilibrium
How does one define ‘where a reaction is at’ when it comes to equilibrium? Chemists often refer to the ‘position’ of equilibrium, although that again implies (terribly) some physical location. Perhaps better words might have been ‘situation’ or ‘condition’, or ‘state’.
Whichever word you use, keep in mind that what is meant is the relative amounts of ‘reactant species’ and ‘product species’. [See how hard it is to get away from the jargon!]
With regard to our example reaction, the most precise (but perhaps long-winded) way of saying this to define the relative amounts of the acetate ions and acetic acid molecules:
Whichever word you use, keep in mind that what is meant is the relative amounts of ‘reactant species’ and ‘product species’. [See how hard it is to get away from the jargon!]
With regard to our example reaction, the most precise (but perhaps long-winded) way of saying this to define the relative amounts of the acetate ions and acetic acid molecules:
This ratio increases with net reaction ‘to the right’. We can imagine the numerical value of this ratio changing from 0 to infinity from a reaction mixture containing only acetic acid molecules (and no acetate ions) to when it hypothetically contains only acetate ions and no acetic acid molecules.
Position of equilibrium 'shifts to the left' or 'shifts to the right'
Firstly, it is important to be aware that the “reactants” and “products” are not in different compartments (and certainly not in two different vessels) – they are all dispersed uniformly throughout the one reaction mixture. The terms “left” and “right” refer to the positions of the formulas in the equation – and have nothing to do with the positions of the species in the reaction mixture.
This jargon concerns a reaction which was at equilibrium, and the equilibrium was “perturbed” (by change of concentrations or of temperature).
After the “perturbation”, there will be net reaction (because the opposite reactions are not happening at the same rate) in the direction such that the reaction mixture comes to equilibrium again.
Consider the reversible reaction represented by
This jargon concerns a reaction which was at equilibrium, and the equilibrium was “perturbed” (by change of concentrations or of temperature).
After the “perturbation”, there will be net reaction (because the opposite reactions are not happening at the same rate) in the direction such that the reaction mixture comes to equilibrium again.
Consider the reversible reaction represented by
After a perturbation of equilibrium, a “shift to the right” means that as the reaction comes to equilibrium again, there is net reaction in the direction that increases the concentrations of the species written on the right side of the equation (acetate ions and hydronium ions), and reduces the concentration of the species written on the left (acetic acid molecules).
Another way (my preferred way) is to say this is that there is an increase in the numerical value of the ratio
Another way (my preferred way) is to say this is that there is an increase in the numerical value of the ratio
Q. Which of the two opposite reactions is happening faster during this net reaction?
Correspondingly, a shift to the “left” means that the net reaction that happens brings about an increase in the concentrations of the species written on the left side of the equation, and a decrease of those written on the left side.
Spontaneous
In everyday life, the term spontaneous refers to something which happens without prompting, immediately and rapidly. If you let go of a ball, it spontaneously falls to the ground.
When we are discussing reversible reactions and chemical equilibrium, the word has an entirely different meaning – unrelated to immediacy or rapidity.
In this context, the word spontaneous refers to a particular “direction” of net reaction.
The spontaneous direction of net reaction is that “direction” of net reaction which would take toward equilibrium a reaction mixture that is not at equilibrium – regardless of how fast (or slow) that might happen.
For example, imagine a reaction mixture in which the reaction represented by the following equation is at equilibrium:
When we are discussing reversible reactions and chemical equilibrium, the word has an entirely different meaning – unrelated to immediacy or rapidity.
In this context, the word spontaneous refers to a particular “direction” of net reaction.
The spontaneous direction of net reaction is that “direction” of net reaction which would take toward equilibrium a reaction mixture that is not at equilibrium – regardless of how fast (or slow) that might happen.
For example, imagine a reaction mixture in which the reaction represented by the following equation is at equilibrium:
And into that solution we add a highly concentrated aqueous sodium acetate solution. At that instant, the concentration of aquated acetate ions in solution would be greater than before (when at equilibrium), so the reaction would no longer be at equilibrium.
But the reaction mixture would again come to an equilibrium condition (different from the original one) by means of net reaction to decrease the concentration of acetate ions, and to increase the concentration of acetic acid molecules.
Then this net reaction “to the left” is the spontaneous direction of net reaction. Net reaction in the reverse direction would take the reaction mixture even further from equilibrium.
But now let’s imagine a vessel containing the gases hydrogen and oxygen. With respect to the reaction represented by
But the reaction mixture would again come to an equilibrium condition (different from the original one) by means of net reaction to decrease the concentration of acetate ions, and to increase the concentration of acetic acid molecules.
Then this net reaction “to the left” is the spontaneous direction of net reaction. Net reaction in the reverse direction would take the reaction mixture even further from equilibrium.
But now let’s imagine a vessel containing the gases hydrogen and oxygen. With respect to the reaction represented by
this mixture is not at equilibrium. This reaction has a very large equilibrium constant, and equilibrium would only be achieved if there were sufficient reaction that essentially none of either the hydrogen or oxygen remains unreacted.
But at ambient temperatures, the reaction is infinitely slow, so that reaction is never achieved (unless the mixture, or part of it, is heated).
Nevertheless, the direction of net reaction that would take this mixture to equilibrium is for net reaction to proceed to form water vapour.
Its infinite slowness does not change this categorization (unlike the meaning of spontaneous in an everyday sense).
If we were to use anthropomorphic language, which I often do (although many say we should not when talking about chemical reactions), we might say that reaction to form water is what this reaction mixture “wants” to do – regardless of whether it is able to, or not.
But at ambient temperatures, the reaction is infinitely slow, so that reaction is never achieved (unless the mixture, or part of it, is heated).
Nevertheless, the direction of net reaction that would take this mixture to equilibrium is for net reaction to proceed to form water vapour.
Its infinite slowness does not change this categorization (unlike the meaning of spontaneous in an everyday sense).
If we were to use anthropomorphic language, which I often do (although many say we should not when talking about chemical reactions), we might say that reaction to form water is what this reaction mixture “wants” to do – regardless of whether it is able to, or not.
A simple analogy: If we have water in a dam on the side of a hill. The spontaneous direction of flow is downhill, even though it cannot do that.
A final reminder
Although chemical equations that we use to talk about a reaction imply some directionality (and so does our jargon), chemical reactions do not have directionality of location.
For good understanding of the meaning of the jargon, visualize what the species in the reaction mixture are doing (see Module 1101 Visualising dynamic chemical equilibrium), rather than the chemical equation.
For good understanding of the meaning of the jargon, visualize what the species in the reaction mixture are doing (see Module 1101 Visualising dynamic chemical equilibrium), rather than the chemical equation.
And now .....
I hope that you are now linguistically well-armed to explore the concepts related to dynamic chemical equilibrium, commencing with Module 1103 Equilibrium constants: The law of equilibrium.
For more discussion about the importance of correct language use in chemistry, especially from a teaching/learning perspective, see Teachers' Corner, Chapter T01.
SELF CHECK - A thinking task
Fill in the gaps in the text
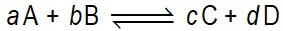
Consider a chemical reaction described by the generalised equation
Presume that all of A, B, C, and D are molecular species (just so that we don’t have to continually refer to them as atoms/ions/molecules).
Fill in the gaps in the following text, using the terms listed here in alphabetical order. Terms in quotation marks are those that imply change of location of the reaction or the reaction species. Prof Bob abhors them for their possible effects on the quality of students’ visualisaton (but recognises that they are part of the entrenched jargon).
Recommendation: Rather than peruse or scan by eye, randomly choosing target terms that seem to (more or less) suit, take the challenge of allocating every term to its appropriate gap. Perhaps take a snip or screenshot of the incomplete text, paste into a document, and print it out for filling in gaps by pen.
Fill in the gaps in the following text, using the terms listed here in alphabetical order. Terms in quotation marks are those that imply change of location of the reaction or the reaction species. Prof Bob abhors them for their possible effects on the quality of students’ visualisaton (but recognises that they are part of the entrenched jargon).
Recommendation: Rather than peruse or scan by eye, randomly choosing target terms that seem to (more or less) suit, take the challenge of allocating every term to its appropriate gap. Perhaps take a snip or screenshot of the incomplete text, paste into a document, and print it out for filling in gaps by pen.
List of terms
C and D, changing, concentrations, [D]/[A], decreasing, double, dynamic, equation, ‘forward direction’, increase, ‘left’, molecules, net, net reaction, no longer, perturbation, ‘position’, products, rate, ratio, reaction, reaction mixture, reaction species, ‘reverse’, reversible, ‘shift to the right’, simultaneous, spontaneous, substances, ‘wants’, zero
C and D, changing, concentrations, [D]/[A], decreasing, double, dynamic, equation, ‘forward direction’, increase, ‘left’, molecules, net, net reaction, no longer, perturbation, ‘position’, products, rate, ratio, reaction, reaction mixture, reaction species, ‘reverse’, reversible, ‘shift to the right’, simultaneous, spontaneous, substances, ‘wants’, zero
The holey text
When the reaction mixture comes to a condition of chemical equilibrium, there are no further changes in the ……………. of any of the reaction species A, B, C, and D. This does not mean that no …….….. is happening. Rather, this is because the …….. at which C and D molecules are increasing (by reaction of A and B) is the same as the rate at which C and D molecules are ………… (to form A and B).
Because both of these reactions are happening at the same time, they are said to be …………….. Such a reaction is said to be a ……….. reaction, and is denoted by the use of a ………… arrow in the chemical equation. In any …………….., this reaction is not necessarily at equilibrium, but it is capable of coming to equilibrium. Because reactions are happening when the mixture is at equilibrium, this is called a condition of ………… chemical equilibrium.
When the reaction mixture comes to a condition of chemical equilibrium, there are no further changes in the ……………. of any of the reaction species A, B, C, and D. This does not mean that no …….….. is happening. Rather, this is because the …….. at which C and D molecules are increasing (by reaction of A and B) is the same as the rate at which C and D molecules are ………… (to form A and B).
Because both of these reactions are happening at the same time, they are said to be …………….. Such a reaction is said to be a ……….. reaction, and is denoted by the use of a ………… arrow in the chemical equation. In any …………….., this reaction is not necessarily at equilibrium, but it is capable of coming to equilibrium. Because reactions are happening when the mixture is at equilibrium, this is called a condition of ………… chemical equilibrium.
It is common for people to refer to substances A and B as the reactants, and C and D as the …………. However, the initial reaction mixture can be made not only by mixing A and B, but also by mixing C and D, or A and C and D, or all of A and B and C and D. The jargon is entrenched, but it can be useful to think of the molecules of all of the substances as …………………. .
It is often said that the reaction between species A and B to form species C and D goes in the ……………………., and that the reaction in the ‘opposite’ direction (between C and D to form A and B) goes in the ………… direction. However, there is no change of location of the reaction or the species. Directionality is only relative to the way that the chemical ………… for the reaction is expressed.
It is often said that the reaction between species A and B to form species C and D goes in the ……………………., and that the reaction in the ‘opposite’ direction (between C and D to form A and B) goes in the ………… direction. However, there is no change of location of the reaction or the species. Directionality is only relative to the way that the chemical ………… for the reaction is expressed.
Any change in the relative amounts of A and B, compared with the amounts of C and D, is referred to as ……… reaction. When the reaction mixture comes to equilibrium, there is ………… net reaction.
Sometimes people use the term …………… of equilibrium to refer to the relative concentrations of A and B, compared with the concentrations of C and D. Net reaction that results in increase of the concentrations of C and D, and decrease of the concentrations of A and B, is sometimes referred to as a …………………... A more correct way of referring to this change would be “net reaction that brings about a decrease in the ………… of the concentration of A compared with that of C – or an …………… in any of the ratios [C]/[A], [C]/[B], [D]/[A], and …………..”
Sometimes people use the term …………… of equilibrium to refer to the relative concentrations of A and B, compared with the concentrations of C and D. Net reaction that results in increase of the concentrations of C and D, and decrease of the concentrations of A and B, is sometimes referred to as a …………………... A more correct way of referring to this change would be “net reaction that brings about a decrease in the ………… of the concentration of A compared with that of C – or an …………… in any of the ratios [C]/[A], [C]/[B], [D]/[A], and …………..”
If a reaction mixture is at equilibrium, and either the concentrations of A, B, C and/or D are changed, or the temperature, is changed the reaction mixture will …………. be at equilibrium. This change is said to be a ………………. of the equilibrium condition.
The chemical equation presented above should not be interpreted as one molecule of A reacting with one molecule of B to form one molecule of C and one molecule of D. Rather it means that the ……………. A and B are ……………. (perhaps by redistribution of their atoms) into the substances C and D. We can imagine that at every moment, many …………… of A and B are colliding with each other, as are many molecules of ……………. If more C and D molecules change into A and B molecules per second (that is the rate of this reaction) is higher than the rate of the reaction A and B molecules to from C and D molecules) there will be ……………… to the ………. This will only happen if this net reaction must occur for the reaction mixture to come to equilibrium.
The chemical equation presented above should not be interpreted as one molecule of A reacting with one molecule of B to form one molecule of C and one molecule of D. Rather it means that the ……………. A and B are ……………. (perhaps by redistribution of their atoms) into the substances C and D. We can imagine that at every moment, many …………… of A and B are colliding with each other, as are many molecules of ……………. If more C and D molecules change into A and B molecules per second (that is the rate of this reaction) is higher than the rate of the reaction A and B molecules to from C and D molecules) there will be ……………… to the ………. This will only happen if this net reaction must occur for the reaction mixture to come to equilibrium.
If net reaction of A and B to form C and D would result in a reaction mixture coming closer to chemical equilibrium, this is called the ………………. ‘direction’ of reaction. Speaking anthropomorphically, this is the ‘direction’ that the reaction …………. to go. Knowing which is the spontaneous ‘direction’ of reaction tells us nothing about how fast the net reaction takes the reaction mixture toward equilibrium.
THE REAL THING (No peeking until you have had a go, or your computer will explode)
When the reaction mixture comes to a condition of chemical equilibrium, there are no further changes in the concentrations of any of the reaction species A, B, C, and D. This does not mean that no reaction is happening. Rather, this is because the rate at which C and D molecules are increasing (by reaction of A and B) is the same as the rate at which C and D molecules are decreasing (to form A and B).
Because both of these reactions are happening at the same time, they are said to be simultaneous. Such a reaction is said to be a reversible reaction, and is denoted by the use of a double arrow in the chemical equation. In any reaction mixture, this reaction is not necessarily at equilibrium, but it is capable of coming to equilibrium. Because reactions are happening when the mixture is at equilibrium, this is called a condition of dynamic chemical equilibrium.
Because both of these reactions are happening at the same time, they are said to be simultaneous. Such a reaction is said to be a reversible reaction, and is denoted by the use of a double arrow in the chemical equation. In any reaction mixture, this reaction is not necessarily at equilibrium, but it is capable of coming to equilibrium. Because reactions are happening when the mixture is at equilibrium, this is called a condition of dynamic chemical equilibrium.
It is common for people to refer to substances A and B as the reactants, and C and D as the products. However, the initial reaction mixture can be made not only by mixing A and B, but also by mixing C and D, or A and C and D, or all of A and B and C and D. The jargon is entrenched, but it can be useful to think of the molecules of all of the substances as reaction species.
It is often said that the reaction between species A and B to form species C and D goes in the ‘forward direction’, and that the reaction in the ‘opposite’ direction (between C and D to form A and B) goes in the ‘reverse’ direction. However, there is no change of location of the reaction or the species. Directionality is only relative to the way that the chemical equation for the reaction is expressed.
It is often said that the reaction between species A and B to form species C and D goes in the ‘forward direction’, and that the reaction in the ‘opposite’ direction (between C and D to form A and B) goes in the ‘reverse’ direction. However, there is no change of location of the reaction or the species. Directionality is only relative to the way that the chemical equation for the reaction is expressed.
Any change in the relative amounts of A and B, compared with the amounts of C and D, is referred to as net reaction. When the reaction mixture comes to equilibrium, there is zero net reaction.
Sometimes people use the term ‘position’ of equilibrium to refer to the relative concentrations of A and B, compared with the concentrations of C and D. Net reaction that results in increase of the concentrations of C and D, and decrease of the concentrations of A and B, is sometimes referred to as a ‘shift to the right’. A more correct way of referring to this change would be “net reaction that brings about a decrease in the ratio of the concentration of A compared with that of C – or an increase in any of the ratios [C]/[A], [C]/[B], [D]/[A], and [D]/[A]”
Sometimes people use the term ‘position’ of equilibrium to refer to the relative concentrations of A and B, compared with the concentrations of C and D. Net reaction that results in increase of the concentrations of C and D, and decrease of the concentrations of A and B, is sometimes referred to as a ‘shift to the right’. A more correct way of referring to this change would be “net reaction that brings about a decrease in the ratio of the concentration of A compared with that of C – or an increase in any of the ratios [C]/[A], [C]/[B], [D]/[A], and [D]/[A]”
If a reaction mixture is at equilibrium, and either the concentrations of A, B, C and/or D are changed, or the temperature, is changed the reaction mixture will no longer be at equilibrium. This change is said to be a perturbation of the equilibrium condition.
The chemical equation presented above should not be interpreted as one molecule of A reacting with one molecule of B to form one molecule of C and one molecule of D. Rather it means that the substances A and B are changing (perhaps by redistribution of their atoms) into the substances C and D. We can imagine that at every moment, many molecules of A and B are colliding with each other, as are many molecules of C and D. If more C and D molecules change into A and B molecules per second (that is the rate of this reaction) is higher than the rate of the reaction A and B molecules to from C and D molecules) there will be net reaction to the ‘left’. This will only happen if this net reaction must occur for the reaction mixture to come to equilibrium.
The chemical equation presented above should not be interpreted as one molecule of A reacting with one molecule of B to form one molecule of C and one molecule of D. Rather it means that the substances A and B are changing (perhaps by redistribution of their atoms) into the substances C and D. We can imagine that at every moment, many molecules of A and B are colliding with each other, as are many molecules of C and D. If more C and D molecules change into A and B molecules per second (that is the rate of this reaction) is higher than the rate of the reaction A and B molecules to from C and D molecules) there will be net reaction to the ‘left’. This will only happen if this net reaction must occur for the reaction mixture to come to equilibrium.
If net reaction of A and B to form C and D would result in a reaction mixture coming closer to chemical equilibrium, this is called the spontaneous ‘direction’ of reaction. Speaking anthropomorphically, this is the ‘direction’ that the reaction ‘wants’ to go. Knowing which is the spontaneous ‘direction’ of reaction tells us nothing about how fast the net reaction takes the reaction mixture toward equilibrium.
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.