Module 2701
Is Earth in energy balance?
What is meant by the term "energy balance" of Earth?
And if Earth is in a state of energy balance, so what?
And if it is not?
Why would it not be?
And if Earth is in a state of energy balance, so what?
And if it is not?
Why would it not be?
The notion of energy balance of Earth is fundamental to all of the discussions in this chapter.
Prof. Bob gives his class .......
Key ideas
At any time, half of the surface of Earth is illuminated: it is receiving radiations (mostly visible light) from the sun. Of course, as Earth revolves, that half which is illuminated gradually changes, over a 24-hour cycle. Something to do with day and night, I believe .......
Image by Arek Socha from Pixabay
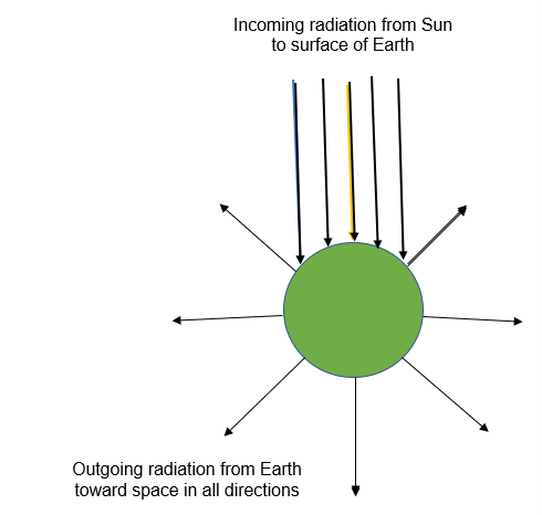
And all the time, energy is being emitted from all over Earth's surface toward space, in all directions. Here is a simplified picture:
Energy balance
If the amount of incoming energy (per second) is the same as the amount of outgoing energy (per second), Earth is in a state of energy balance. There is no loss or gain of energy. The temperature of the crust and the atmosphere does not change.
If the amount of incoming energy (per second) is the same as the amount of outgoing energy (per second), Earth is in a state of energy balance. There is no loss or gain of energy. The temperature of the crust and the atmosphere does not change.
Earth is in a state of energy balance, and the temperature does not change, if
Rate of outgoing radiation = rate of incoming radiation
Rate of outgoing radiation = rate of incoming radiation
Energy out of balance ...
But if the rate of energy arriving on Earth from the sun is (for whatever reason) greater than the rate of loss of energy from Earth, the energy of Earth will increase, and the temperature will increase.
And, conversely, if the rate of loss of energy from Earth is greater than the rate of energy falling upon Earth, the energy will decrease, and the temperature will decrease.
Which brings us to the question: If the rate of incoming radiation from the sun is constant (as iit is), how can we explain that the temperature of the surface of Earth is increasing?
SELF CHECK - Have you got it?
Is Earth in balance (energetically)? What could cause energy balance to be disrupted?
Suppose that Earth is in a state of energy balance. You have a super power that allows you to dial up either an increase or decrease in the rate of incoming radiation, or an increase or decrease in the rate of outgoing radiation.
1. Which actions would cause the temperature to rise?
2. Which actions would cause the temperature to fall?
3. (Foreshadowing) Which of the above (although not activated by you) do you think is bringing about global warming?
2. Which actions would cause the temperature to fall?
3. (Foreshadowing) Which of the above (although not activated by you) do you think is bringing about global warming?
ANSWERS
1. Either increase the rate of incoming radiation, or decrease the rate of outgoing radiation.
2. Either decrease the rate of incoming radiation, or increase the rate of outgoing radiation.
3. "Greenhouse gases" decrease the rate of outgoing radiation. See Module 2705 The "greenhouse effect".
2. Either decrease the rate of incoming radiation, or increase the rate of outgoing radiation.
3. "Greenhouse gases" decrease the rate of outgoing radiation. See Module 2705 The "greenhouse effect".
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.