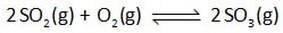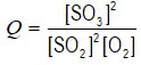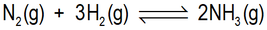Module 0505
Chemical equations: What can they tell us?
What information is provided by balanced chemical equations?
What do they not tell us?
Starting point: What is a balanced chemical equation?
Before you begin, be clear about the difference between a chemical reaction and a chemical equation (see Module 050 Chemical reactions, chemical equations).
And the general discussion in this module only applies if we are talking about balanced chemical equations:
- The formulas of substances and species in the equation accurately represent the the compositions of the reactants and products.
- When reaction happens, the total number of atoms of each element in the products is the same as in the reactants: that there is no implication that atoms of any element are either created, nor 'lost'.
- At all times, the reaction mixture remains electrically neutral: the sum of the charges on the formulas of the reactants is zero, as is the sum of the charges on the formulas of the products.
A balanced chemical equation that is used to represent a chemical reaction (a real process) can provide us with important information about that reaction. But be careful not to read from it more than is valid to do so. This is fundamental to learning chemistry.
Watch the video, and all will become clear about what balanced chemical equations do (and don't) tell us .....
Prof Bob and Aussie come to Aha! moments about the information available from balanced chemical equations.
KEY IDEAS - Interpreting chemical equations
What can balanced chemical equations can tell us?
Balanced chemical equations can tell us the following:
- What the reactant substances are, and what the product substances are.
- For however much reaction happens, the relative chemical amounts (in moles) of the reactant substances that are “consumed” and product substances that are produced.
Chemical equations tell us about relative chemical amounts of substances, and not about actual (absolute) amounts that reacted.
Not sure what is meant by the term chemical amount? See the module 0501 Chemical amount and its unit of measurement, the mole.
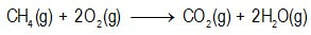
Take, for example, the balanced chemical equation for the combustion of methane (natural gas):
This equation tells us that for every 1 mol of the gaseous substance methane that reacts, 2 mol of the substance oxygen gas also reacts and the resultant products are 1 mol of the gaseous substance carbon dioxide and 2 mol of the substance water vapour. It does not tell us that 1 mol of methane reacted (nor that one molecule reacted).
This relationship applies, regardless of how much methane actually reacts.
The equation enables us to calculate, for example, the chemical amount (or weight) of gaseous carbon dioxide that is formed if a specified chemical amount (or weight) of methane is burned. This is the field of stoichiometry.
Chemical equations tell us about relative chemical amounts of substances, and not about relative numbers of atoms or molecules or ions – although the relative numbers of these can often be deduced. Although the relative numbers of molecules of those substance are the same as the relative amounts of the substances, the equation should not be taken as a portrayal (leading to a mental image) of 2 molecules of oxygen reacting with 1 molecule of methane.
[We use equations with different meanings when we consider the interaction of individual molecules (or atoms or ions) of reactants when we discuss the mechanisms of reactions (How they happen: what molecules need to collide, which bonds break, which bonds are formed ..... ). On these occasions, we focus on single collisions between molecules (of the trillions upon trillions of collisions that happen during a reaction), and the formulas in the equation represent single molecules.]
This relationship applies, regardless of how much methane actually reacts.
The equation enables us to calculate, for example, the chemical amount (or weight) of gaseous carbon dioxide that is formed if a specified chemical amount (or weight) of methane is burned. This is the field of stoichiometry.
Chemical equations tell us about relative chemical amounts of substances, and not about relative numbers of atoms or molecules or ions – although the relative numbers of these can often be deduced. Although the relative numbers of molecules of those substance are the same as the relative amounts of the substances, the equation should not be taken as a portrayal (leading to a mental image) of 2 molecules of oxygen reacting with 1 molecule of methane.
[We use equations with different meanings when we consider the interaction of individual molecules (or atoms or ions) of reactants when we discuss the mechanisms of reactions (How they happen: what molecules need to collide, which bonds break, which bonds are formed ..... ). On these occasions, we focus on single collisions between molecules (of the trillions upon trillions of collisions that happen during a reaction), and the formulas in the equation represent single molecules.]
What can balanced chemical equations NOT tell us?
Balanced chemical equations cannot tell us any of the following:
Balanced chemical equations cannot tell us any of the following:
- How much reaction actually happened in any situation – that is, how many moles of substances reacted, or were formed. It can only tell us about relative chemical amounts.
- How much of each reactant species was in the reaction chamber initially.
- Whether the reaction that it represents is exothermic or endothermic.
- How fast the reaction represented proceeds (that is, its rate of reaction – how many moles of substances react per unit of time).
- Anything about the reaction mechanism (that is, the sequence of events that happen at the sub-microscopic level).
In summary .......
A balanced reaction equation tells us only what the substances are that react and are formed, and the relative chemical amounts (in moles) that react or are formed.
Addendum
Neither can a balanced chemical equation tell us anything about how far a reaction goes before coming to equilibrium.
It allows us to deduce the form of the reaction quotient, Q, whose value at equilibrium (at defined temperature) is called the equilibrium constant, K (Module 1103 Equilibrium constants: The law of equilibrium). But it can tell us nothing about the magnitude of K.
For example, for the reaction represented by the equation
we can deduce from the balanced equation that the mathematical expression of reagent concentrations that we call the reaction quotient, Q, is
The numerical value of Q is the same in all reaction mixtures in which this reaction is at equilibrium (at the same temperature) – which is not true of any other function of the concentrations.
However, the balanced chemical equation can give us no sense at all of the value of Q in reaction mixtures at equilibrium (that is, of the magnitude of the equilibrium constant, K).
However, the balanced chemical equation can give us no sense at all of the value of Q in reaction mixtures at equilibrium (that is, of the magnitude of the equilibrium constant, K).
Addendum 2
In mathematics, an equation expresses an equality: whatever is on the left side of the equation has the same numerical value as that on the right side.
Balanced chemical equations are not like that: the stuffs on the left are quite different from those on the right. The stuffs on the left disappear, and the stuffs on the right appear (as a result of breaking and forming bonds).
This is the main objective of chemical equations. Nonetheless, there is one sense of equality in a balanced chemical equation - and that is that, consistent with the law of conservation of atoms (also called the law of conservation of matter) atoms neither disappear nor form.
Rather, they re-arrange themselves to form different stuffs. But the number of atoms of each element in the reacting stuffs (left side) is the same in the product stuffs (right side). You can't have some atoms of an element left over.
And of course, this means that the total mass of stuffs that react must be equal to the total mass of products formed. This is called the law of conservation of mass.
[Beware! In the otherwise excellent article that you may find from the link above, in reference to the chemical equation for photosynthesis is the statement: "This equation says that six carbon dioxide molecules combine with six water molecules to form one sugar molecule and six molecules of oxygen". No it doesn't! Reaction is not limited to just six molecules - many, many, more. This should have been expressed as "This equation says that for every six carbon dioxide molecules that combine with six water molecules (regardless of how many do so), one sugar molecule and six molecules of oxygen are formed". Even better, since the reactants and products are stuffs: "This equation says that for every six moles of carbon dioxide that combine with six moles of water, one mole of sugar and six moles of oxygen are formed". Being aware of this profound distinction will be of great benefit to the deep understanding of anyone learning Chemistry.]
SELF CHECK: Some thinking tasks
Which of the following statements about reaction of nitrogen gas and hydrogen gas to form ammonia gas can be deduced from the balanced chemical equation shown here?
A The initial amounts of reactants are 1 mol of ammonia gas and 3 mol of hydrogen gas.
B Regardless of how much nitrogen gas, hydrogen gas and ammonia gas are in a reaction vessel initially, if 0.6 mol of hydrogen reacts with nitrogen, 0.4 mol of ammonia is produced.
C The reaction happens as a result of collisions between one nitrogen molecule and three hydrogen molecules simultaneously.
D Reaction to form ammonia is exothermic.
E The reaction is slow because the likelihood of simultaneous collision of one nitrogen molecule and three hydrogen molecules is very low.
F If, over a period of time, the mount of ammonia gas in a reaction vessel increases by 0.6 mol, in that time the amount of hydrogen gas decreases by 0.9 mol.
G At equilibrium, the “backward” reaction of ammonia to form nitrogen and hydrogen occurs as a result of collisions each involving two ammonia molecules.
H The amounts of reactant species initially present are: 1 mol of nitrogen, 3 mol of hydrogen, and 2 mol of ammonia.
I The amounts of reactant species present when the reaction reaches equilibrium are: 1 mol of nitrogen, 3 mol of hydrogen, and 2 mol of ammonia.
J Because the reaction, the basis of the Haber process, is used to produce ammonia fertiliser that can dramatically increase crop production, it is regarded as one of the most important reactions known.
B Regardless of how much nitrogen gas, hydrogen gas and ammonia gas are in a reaction vessel initially, if 0.6 mol of hydrogen reacts with nitrogen, 0.4 mol of ammonia is produced.
C The reaction happens as a result of collisions between one nitrogen molecule and three hydrogen molecules simultaneously.
D Reaction to form ammonia is exothermic.
E The reaction is slow because the likelihood of simultaneous collision of one nitrogen molecule and three hydrogen molecules is very low.
F If, over a period of time, the mount of ammonia gas in a reaction vessel increases by 0.6 mol, in that time the amount of hydrogen gas decreases by 0.9 mol.
G At equilibrium, the “backward” reaction of ammonia to form nitrogen and hydrogen occurs as a result of collisions each involving two ammonia molecules.
H The amounts of reactant species initially present are: 1 mol of nitrogen, 3 mol of hydrogen, and 2 mol of ammonia.
I The amounts of reactant species present when the reaction reaches equilibrium are: 1 mol of nitrogen, 3 mol of hydrogen, and 2 mol of ammonia.
J Because the reaction, the basis of the Haber process, is used to produce ammonia fertiliser that can dramatically increase crop production, it is regarded as one of the most important reactions known.
.
.
.
.
.
.
.
Answer: B and F only. Recall that you were asked about what can be deduced from the equation - and not which statements are correct.
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.
knowledge + wisdom → empowerment to do worthwhile things
(Prof Bob)