Module 0902
Miscibility of liquids in each other
Which liquids dissolve in each other? Which do not? Why?
"Like dissolves like"?
Competing 'driving forces' - entropy and energy
Prof Bob's law of miscibility
The video here (and some in other modules) is long-ish. If you just want a superficial understanding of the concepts, perhaps a glossary entry, or even a common half-page textbook description built upon the saying "Like dissolves like", is for you. If you want to understand, .... have a look at Prof Bob's video .......
Prof Bob apologises: (i) Near the end of the video (19:40) two seconds have been lost. In the lost bit, Aussie said "The energy required to separate both sorts of molecules, and the energy we get back, are quite different." (ii) He can't spell "miscibility"! Did you notice?
KEY IDEAS - Miscibility of liquids in each other
Unlock the door to the concepts that make sense of the phenomenon of miscibility of pairs of liquids, and immiscibility of others.
Some chemistry jargon …..
Liquids that dissolve in each other are said to be miscible (a variation of mixable). If they are not miscible (instead, they remain in two separate layers, with the most dense liquid the bottom layer) they are immiscible.
Liquids that dissolve in each other are said to be miscible (a variation of mixable). If they are not miscible (instead, they remain in two separate layers, with the most dense liquid the bottom layer) they are immiscible.
Visualization:
Keep in mind that the liquids that we most commonly encounter (water, ethanol, hexane, octane, ….) are molecular substances: they are composed of molecules.
So when we come to explanations for miscibility/immiscibility, it will be necessary to visualize molecules rather closely packed, but able to move past each other. There are forces of attraction between the molecules, although these don’t keep them “frozen” unless the temperature falls below the freezing point (when they become solids).
They make many collisions, so do not move far, in comparison with the rapid translational movement of molecules in gases.
Prof Bob's law of miscibility: Competing "driving forces"
There is a commonly expressed guideline: “Like dissolves like” - meaning that (i) polar liquids are miscible in each other, (ii) non-polar liquids are miscible in each other, but (iii) a polar liquid and a non-polar liquid are immiscible.
However, this generalisation is not always correct. In any case, while it is a useful predictive guideline, it is not an explanation: it provides no answers to the question “Why?”
Prof Bob’s law of miscibilities of molecular liquids in each other: There is a natural tendency for the molecules of different liquids to be scrambled among each other (that is, for the liquids to be miscible in each other). Forces of attraction between the molecules in each of the pure liquids may constrain them from doing so.
This “natural tendency” to mix among each other is matter of probability: It is infinitely more probable that molecules will disperse among each other, rather than remain separated - if there is nothing stopping them from doing so. Compare with a box containing lots of red balls and white balls (with plenty of free space). If shaken up, what is the probability that we will find no mixing of the differently coloured balls? Essentially zero.
In fact we are talking about a property called entropy – a measure of the probability of things arranging in a certain way. The higher the probability, the greater the likelihood of being in that arrangement, the higher the entropy.
The scrambling molecules of two liquids among each other is a high entropy condition (is a more probable arrangement) than the molecules remaining in two separate layers.
Increase of entropy favours an event happening, but sometimes there are (energy) factors that do not allow the event to happen.
If there are forces of attraction between molecules, work has to be done to separate them. If the work is done by molecules of one liquid pushing in between other molecules that attract each other, the energy increases.
Chemical interactions don’t like going toward situations that require work to be done (energy increase). By analogy, water doesn’t want to go uphill, even if we can do work (use energy) to get it there.
Weighing up the "driving forces". Which one wins?
Let’s contemplate the miscibility in each other of two liquids, generalised as A and B.
There is a natural tendency for the molecules of A and the molecules of B to scramble (increase of entropy)
But we need to consider the following energy contributions:
- The force of attraction between A molecules in pure A – because energy must be used to separate them from each other to insert B molecules between them
- The force of attraction between B molecules in pure B – because energy must be used to separate them if they are to intersperse among the A molecules
- The force of attraction between A molecules and B molecules when they are scrambled among each other
Factors 1. and 2. provide resistance to scrambling of the molecules: each of them would bring about an increase in energy.
Some energy compensation (lowering of energy) will result from the attractions between scrambled A molecules and B molecules.
Increase of entropy says to the molecules “Scramble!”. Increase of energy opposes this tendency: “Don’t scramble!”
There is a tendency when learning chemistry to over-rate our ability to predict outcomes from explanatory models. In fact, we can only weigh up the opposing effects, and provide an explanation, by using the visual evidence of whether the two liquids are miscible or immiscible. That is, we see the phenomenon, and then deduce which are the dominant factors.
If A and B are miscible (that is, the molecules remain scrambled among each other), we can conclude that the entropy factor dominates.
If liquids A and B are immiscible, they will remain as two separate layers. Then we deduce that the energy increase is too great: the A molecules squeeze out the B molecules and spit them out into a separate layer.
Aha!
A particular case: methanol and water
Observation: methanol and water are miscible How do we explain this phenomenon?
There is a natural tendency for methanol molecules and water molecules to be scrambled among each other.
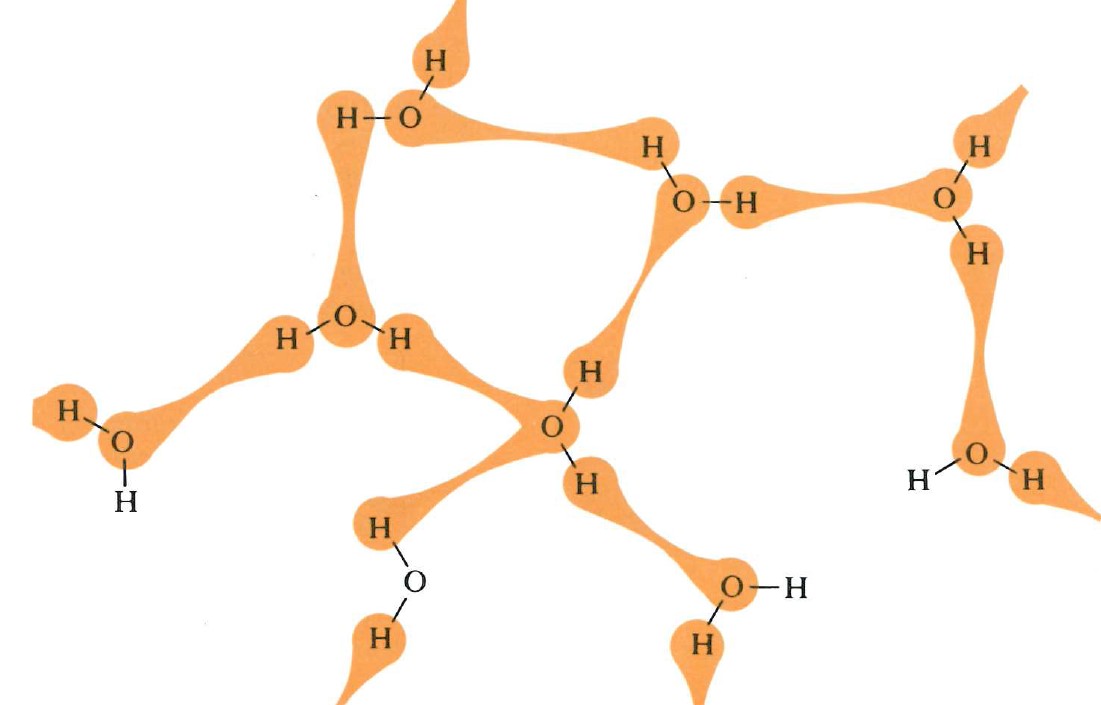
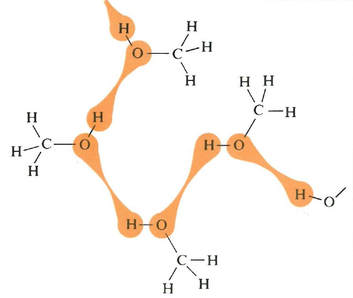
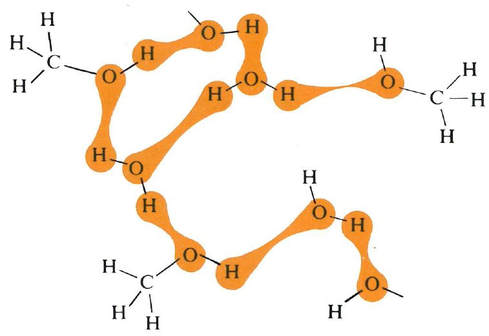
But there is resistance to scrambling due to the need to pull apart the water molecules against the forces of attraction due to hydrogen bonding, as well as by the need to pull apart the methanol molecules – also against attractions due to hydrogen bonding.
Two-dimensional, static representations of molecules in water (left), and methanol (right), indicating the momentary location of hydrogen bonding as the molecules move past each other. Source: Bucat, R.B. (Ed.) Elements of Chemistry: Earth, Air, Fire and Water. Australian Academy of Science, 1984
On the other hand, the resistance to scrambling is lessened by the attractions (again due to hydrogen bonding) between water molecules and methanol molecules.
Source: Bucat, R.B. (Ed.) Elements of Chemistry: Earth, Air, Fire and Water. Australian Academy of Science, 1984
How can we tell if the work needed to be done to pull molecules apart is enough to prevent the natural tendency to scramble?
Easy. Let’s look and see. Aha! They dissolve. So we know that the scrambling has won out over the net resistance due to intermolecular forces.
It is not so easy to weigh up the two opposing tendencies. We can only use this model to explain observed miscibility or immiscibility, rather than to predict – except in obvious cases
Water and hexane: polar and non-polar liquids
Now how do we explain the observation that hexane and water are miscible?
There is a natural tendency for the hexane and water molecules to scramble.
But imagine long hexane molecules pushing in between water molecules. Each hexane molecule would break the hydrogen bonding between several water molecules. This would require a considerable amount of work (demand for energy).
And more work needs to be done to separate the hexane molecules from each other - maybe not a lot more because the only forces of attraction between hexane molecules are weak dispersion forces.
And we get some energy back from the attraction between water molecules and hexane molecules when they come near each other – but hydrogen bonding is not possible, only dispersion forces.
Given the evidence of immiscibility, it seems that the net energy requirement is too much to allow the natural tendency for the molecules to co-mingle.
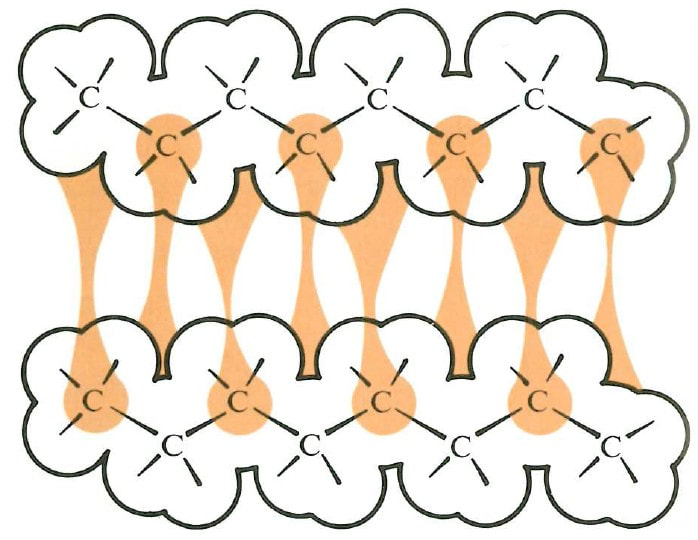
Hexane and octane: Both non-polar
The non-polar hydrocarbons hexane and octane are miscible.
Q. What are the formulas of hexane and octane? It might be useful to your understanding to draw the structures of their molecules.
As ever, co-mingling of the two types of molecule is a highly probable (high entropy) condition (in the absence of energy constraints).
Being non-polar, there are only relatively weak forces of attraction between molecules in both pure hexane and pure octane. So relatively little work needs to be done to separate the molecules in pure hexane in order to scramble them among octane molecules – as well as to separate the molecules in pure octane.
Source: Bucat, R.B. (Ed.) Elements of Chemistry: Earth, Air, Fire and Water. Australian Academy of Science, 1984
We might expect the energy compensation due to dispersion forces between co-mingled hexane molecules and octane molecules to approximately cancel the work done to separate those molecules.
In fact (using a little anthropomorphism) the octane molecules probably don’t even realise that there are hexane molecules around them (rather than more octane molecules as in pure octane), and vice versa.
The energy increase is minimal. So there is only minimal constraint on the natural tendency of the molecules to co-mingle. They do.
Like dissolves like?
This is such a commonly stated guideline. How accurate is it? This is the subject of Module 0903 Like dissolves like? Shades of grey
SELF CHECK: Some thinking tasks
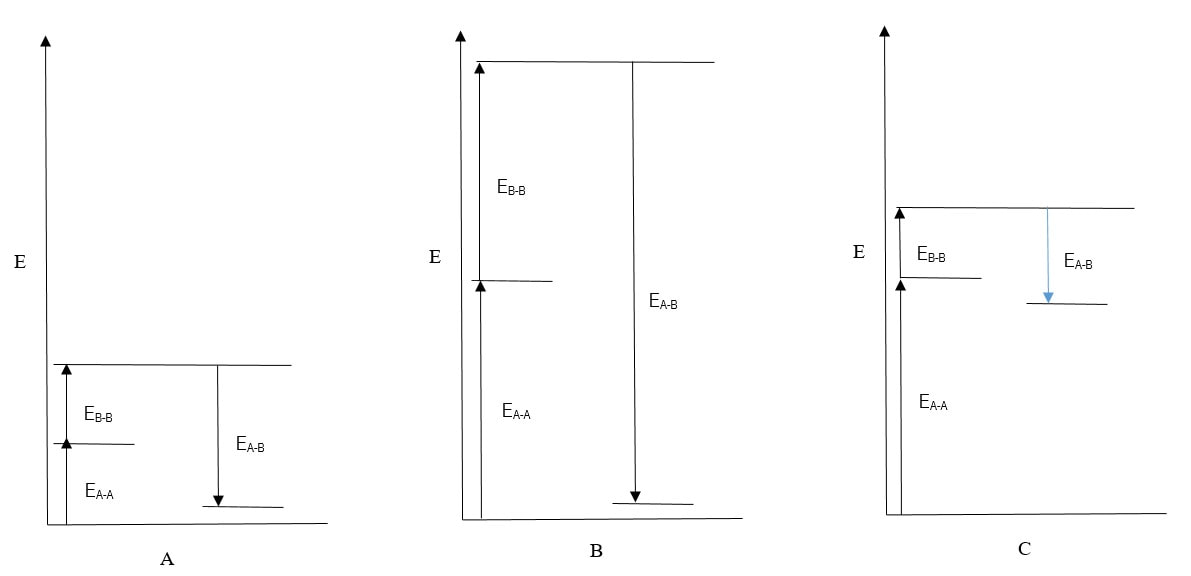
(a) Which of the diagrams best explains the miscibility of two polar liquids?
(b) Which of the diagrams best explains the the immiscibility of a polar liquid with a non-polar liquid?
(c) Which of the diagrams best explains the miscibility of two non-polar liquids?
(b) Which of the diagrams best explains the the immiscibility of a polar liquid with a non-polar liquid?
(c) Which of the diagrams best explains the miscibility of two non-polar liquids?
2. Imagine two adjacent compartments in a large vessel, one containing pure oxygen gas, and the other pure nitrogen gas. The barrier between the compartments is removed. The two gases mix in each other to form a gaseous solution. Which of the following is the best explanation of the phenomenon?
A Co-mingling of molecules occurs because there are strong forces of attraction between the oxygen molecules and nitrogen molecules.
B The observed phenomenon is consistent with Prof Bob’s law of miscibility, the forces of attraction between the gas molecules being so negligible as to provide no resistance to achievement of the highest entropy condition.
C The observed phenomenon is consistent with Prof Bob’s law of miscibility. Although it takes a lot of energy to break apart oxygen molecules to form oxygen atoms, as well as to break apart nitrogen molecules to form nitrogen atoms, the compensating energy due to attraction between oxygen atoms and nitrogen atoms is also large, so that the energy deficiency is small.
D Like dissolves like.
A Co-mingling of molecules occurs because there are strong forces of attraction between the oxygen molecules and nitrogen molecules.
B The observed phenomenon is consistent with Prof Bob’s law of miscibility, the forces of attraction between the gas molecules being so negligible as to provide no resistance to achievement of the highest entropy condition.
C The observed phenomenon is consistent with Prof Bob’s law of miscibility. Although it takes a lot of energy to break apart oxygen molecules to form oxygen atoms, as well as to break apart nitrogen molecules to form nitrogen atoms, the compensating energy due to attraction between oxygen atoms and nitrogen atoms is also large, so that the energy deficiency is small.
D Like dissolves like.
3. Which of the following statements is correct?
A In methanol, the hydrogen bond is the O-H bond in each molecule.
B The stronger is the O-H bond in the molecules, of various substances, the stronger is the force of atraction between its molecules.
C Hydrogen bonding does not refer to bonds within molecules: rather it refers to forces of attraction between molecules.
A In methanol, the hydrogen bond is the O-H bond in each molecule.
B The stronger is the O-H bond in the molecules, of various substances, the stronger is the force of atraction between its molecules.
C Hydrogen bonding does not refer to bonds within molecules: rather it refers to forces of attraction between molecules.
4. Which one of the statements below is correct?
A Butane is insoluble in water, but butanol is soluble in water.
B Both butane and butanol are soluble in water.
C Both butane and butanol are insoluble in water
D Butane is soluble in water, but butanol is insoluble in water.
A Butane is insoluble in water, but butanol is soluble in water.
B Both butane and butanol are soluble in water.
C Both butane and butanol are insoluble in water
D Butane is soluble in water, but butanol is insoluble in water.
5. You buy a new pack of cards. In the box, they are ordered from ace to king, suit by suit (spades, then clubs, then diamonds, then hearts). Before you play a game, you shuffle the cards. Has the entropy of the pack of cards (A) increased, or (B) decreased?
6. Ethylene glycol (ethane 1,2-diol, CH2(OH)CH2(OH)) is miscible with water in all proportions, but immiscible with hexane. Which of the following is/are correct in relation to the miscibility of ethylene glycol and water?
A There is a natural tendency (increase of entropy) for ethylene glycol molecules and water molecules to disperse among each other, and the net energy demand of doing so is not too big to prevent this from happening.
B The main reason why the net energy demand of scrambling ethylene glycol molecules and water molecules is not too big is that the large amount of energy needed to separate ethylene glycol molecules from each other (due to hydrogen bonding) and to separate water molecules from each other (due to hydrogen bonding) is well compensated by strong forces of attraction between water molecules and ethylene glycol molecules (due to hydrogen bonding) when interspersed among each other.
C The main reason why the net energy demand of scrambling ethylene glycol molecules and water molecules is not too big is that there are only weak forces of attraction between ethylene glycol molecules, as well as between water molecules, so little work needs to be done to separate them when the molecules of the two liquids intersperse among each other.
D There are two hydrogen bonds in each ethylene glycol molecule.
7. Ethylene glycol (ethane 1,2-diol, CH2(OH)CH2(OH)) is miscible with water in all proportions, but immiscible with hexane. Which of the following is/are correct in relation to the immiscibility of ethylene glycol and hexane?
A There is a natural tendency (increase of entropy) for ethylene glycol molecules and hexane molecules to disperse among each other, but the net energy demand of doing so is large enough to prevent this from happening.
B The main reason why there is a large net energy demand of scrambling ethylene glycol molecules and hexane molecules among each other is the large amount of work needed to be done to separate hexane molecules from each other, due to hydrogen bonding between them.
C In each molecule of hexane (C6H14) there are 14 hydrogen bonds.
D The main reason why there is a large net energy demand of scrambling ethylene glycol molecules and hexane molecules among each other is that the large amount of work needed to be done to separate ethylene glycol molecules (due to hydrogen bonding between them), as well as some more work to separate hexane molecules (dispersion forces) is not well compensated by forces of attraction between water molecules and ethylene glycol molecules if they are scrambled among each other.
A There is a natural tendency (increase of entropy) for ethylene glycol molecules and hexane molecules to disperse among each other, but the net energy demand of doing so is large enough to prevent this from happening.
B The main reason why there is a large net energy demand of scrambling ethylene glycol molecules and hexane molecules among each other is the large amount of work needed to be done to separate hexane molecules from each other, due to hydrogen bonding between them.
C In each molecule of hexane (C6H14) there are 14 hydrogen bonds.
D The main reason why there is a large net energy demand of scrambling ethylene glycol molecules and hexane molecules among each other is that the large amount of work needed to be done to separate ethylene glycol molecules (due to hydrogen bonding between them), as well as some more work to separate hexane molecules (dispersion forces) is not well compensated by forces of attraction between water molecules and ethylene glycol molecules if they are scrambled among each other.
ANSWERS
1. (a) B; (b) C; (c) A
2. B
3. C
4. A
5. A (increased)
6. A and B. [Hydrogen bonds are forces of attraction between molecules, not within molecules]
7. A and D
1. (a) B; (b) C; (c) A
2. B
3. C
4. A
5. A (increased)
6. A and B. [Hydrogen bonds are forces of attraction between molecules, not within molecules]
7. A and D
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.