Module 2711
Carbon dioxide from our cars. How? How much?
How is carbon dioxide produced in internal combustion engines? Where does it come from?
How much carbon dioxide do our cars produce? Tiny amounts, surely?
Prelude
Hopefully it is not too far into the future before this module is irrelevant because all of our cars will be powered by electric motors, rather than internal combustion engines.
But if you have an electric car, don’t feel too saintly unless you know that the charger you use to replenish the batteries is not drawing its energy from a coal-fired power plant. If it is, then instead of carbon dioxide spewing out of the exhaust of your car, it is spewing out of the smokestacks of the power plant - perhaps far from your home. You have simply shifted the location of the source of carbon dioxide.
This module concerns cars powered by internal combustion engines.
The focus is on how much carbon dioxide is produced by the combustion of the fuel in air. Where the energy comes from during combustion of the fuel is the subject matter of Module 2712 The source of energy come from combustion of fuels.
But if you have an electric car, don’t feel too saintly unless you know that the charger you use to replenish the batteries is not drawing its energy from a coal-fired power plant. If it is, then instead of carbon dioxide spewing out of the exhaust of your car, it is spewing out of the smokestacks of the power plant - perhaps far from your home. You have simply shifted the location of the source of carbon dioxide.
This module concerns cars powered by internal combustion engines.
The focus is on how much carbon dioxide is produced by the combustion of the fuel in air. Where the energy comes from during combustion of the fuel is the subject matter of Module 2712 The source of energy come from combustion of fuels.
Carbon dioxide from combustion of fuels
Internal combustion engines in cars are designed to harness the energy obtained from combustion of fuels (such as petrol, diesel, or natural gas) in order to propel the car.
Combustion is the term for chemical reaction with oxygen.
If the fuel is a compound (or mixture of compounds) whose molecules contain carbon atoms, carbon dioxide is a by-product of the chemical reaction of combustion.
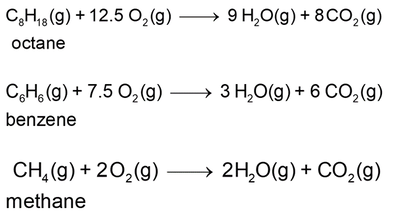
Balanced chemical equations (Module 0505, Chemical equations: what can they tell us?) that represent combustion of various compounds are shown below. All reactants and products are in their gaseous states, as they are during combustion.
Combustion is the term for chemical reaction with oxygen.
If the fuel is a compound (or mixture of compounds) whose molecules contain carbon atoms, carbon dioxide is a by-product of the chemical reaction of combustion.
Balanced chemical equations (Module 0505, Chemical equations: what can they tell us?) that represent combustion of various compounds are shown below. All reactants and products are in their gaseous states, as they are during combustion.
There is no avoiding it: carbon dioxide is always produced by combustion of carbon-based fuels (that is, those fuels whose molecules contain carbon atoms). The only differences are in the amounts of carbon dioxide produced – per mole of fuel, per kilogram of fuel, or per unit of heat energy released.
How much carbon dioxide do our cars produce?
It’s time to get into some stoichiometry – calculations based on balanced chemical reactions (Module 0505 Chemical equations: What can they tell us).
The petrol (or gasoline) that we put into cars is a mixture of many compounds. For our purposes we can presume that the compound octane (C8H18) approximates the behaviour of the mixture.
The petrol (or gasoline) that we put into cars is a mixture of many compounds. For our purposes we can presume that the compound octane (C8H18) approximates the behaviour of the mixture.
The equation for its combustion is shown above. With no need to be overly accurate, we can use the following values of molar mass of the reactants and products:
M(C8H18) = 114 g/mol
M(O2) = 32 g/mol
M(H2O) = 18 g/mol
M(CO2) = 44 g/mol
Then we can calculate the relative masses of substances that react and of the substances that are produced. For example, in the case of octane ……
M(C8H18) = 114 g/mol
M(O2) = 32 g/mol
M(H2O) = 18 g/mol
M(CO2) = 44 g/mol
Then we can calculate the relative masses of substances that react and of the substances that are produced. For example, in the case of octane ……
This tells us that for every 114 g of octane that burns, 352 g of carbon dioxide is formed.
As you can see, the mass of carbon dioxide that is produced is a little more than three times the mass of octane that is burned.
As you can see, the mass of carbon dioxide that is produced is a little more than three times the mass of octane that is burned.
Shall we go for a drive?
Let’s drive our car for 500 km (either in one trip, or in several – that is irrelevant)
Off the top of your head, guess how much carbon dioxide comes out of the exhaust. C'mon ... guess!
Let's see ……
Suppose that our car uses 10 L of fuel per 100 km (It’s a biggish car, being driven rather fast). In 500 km, the car will use up 50 L fuel. Taking the density of petrol as 0.80 kg/L, the mass of fuel used 40 kg.
Off the top of your head, guess how much carbon dioxide comes out of the exhaust. C'mon ... guess!
Let's see ……
Suppose that our car uses 10 L of fuel per 100 km (It’s a biggish car, being driven rather fast). In 500 km, the car will use up 50 L fuel. Taking the density of petrol as 0.80 kg/L, the mass of fuel used 40 kg.
So, the mass of carbon dioxide emitted by the car is a little more than 3 x 40 kg = 120 kg.
Are you surprised?
If that much carbon dioxide were put into a container at 20 °C and 1 bar pressure, it’s volume would be 60 000 L.
Not my car, surely!
What will I do about it?
Are you surprised?
If that much carbon dioxide were put into a container at 20 °C and 1 bar pressure, it’s volume would be 60 000 L.
Not my car, surely!
What will I do about it?
And that was just one car!
Imagine all of the cars, trucks, planes, industries all burning carbon-based fuels all the time ....
The total world combustion of crude oil used for transport and manufacturing purposes annually is about 12 000 000 000 tonnes (12 gigatonnes)
Using our three-times rule, we can estimate that the global emissions of carbon dioxide for energy production is about 36 000 000 000 tonnes.
No wonder that photosynthesis and dissolving in the oceans have trouble keeping up!
The total world combustion of crude oil used for transport and manufacturing purposes annually is about 12 000 000 000 tonnes (12 gigatonnes)
Using our three-times rule, we can estimate that the global emissions of carbon dioxide for energy production is about 36 000 000 000 tonnes.
No wonder that photosynthesis and dissolving in the oceans have trouble keeping up!

The white stuff that we can often see being emitted from factories and power plants is not carbon dioxide. It is probably steam: fine droplets of water. This is not a pollutant. The invisibility of carbon dioxide gas is one of the problems with dealing with it’s increasing concentration. If you can’t see it, does it exist? Just imagine if carbon dioxide was purple!
SELF CHECK - Some thinking tasks
ANSWERS
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.