Module 2710
Doesn’t water vapour absorb all of the emitted infrared radiation?
Water vapour absorbs infrared radiations strongly.
Water vapour is the most important greenhouse gas.
There is much more water vapour in the atmosphere than there is carbon dioxide.
So how can carbon dioxide molecules absorb a significant amount of the infrared radiation emitted from Earth?
Isn’t it all absorbed by water vapour?
The answer is simple. And it is all about specifics, rather than generalities.
Does water vapour absorb infrared radiations?
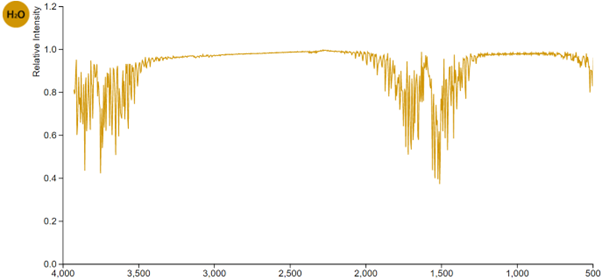
Yes it does. Here is its infrared absorption spectrum – a graph of the relative amount of infrared radiation absorbed at each wavelength in the IR range.
The horizontal axis indicates the frequency of waveforms measured as reciprocal centimetres (1/cm). The lower the wave frequency (to the right), the longer the wavelength.
Source: King’s Centre for Visualization in Science, at https://applets.kcvs.ca/IRWindows/IRWindows.html
Source: King’s Centre for Visualization in Science, at https://applets.kcvs.ca/IRWindows/IRWindows.html
Does water vapour absorb infrared radiations at all wavelengths?
No. It has absorption “bands” – specific ranges of wavelength at which it absorbs strongly.
But, like all substances, there are many wavelengths which it absorbs very poorly, or not at all.
Water vapour absorbs hardly any of the infrared radiations with wavelengths in the range 500 – 1200 nm, nor in the range 2000- 3300 nm.
But, like all substances, there are many wavelengths which it absorbs very poorly, or not at all.
Water vapour absorbs hardly any of the infrared radiations with wavelengths in the range 500 – 1200 nm, nor in the range 2000- 3300 nm.
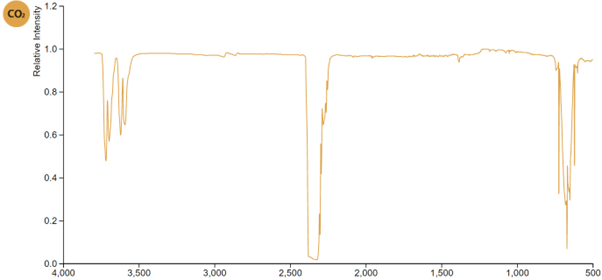
Which infrared wavelengths does carbon dioxide absorb?
Interpret its absorption spectrum …..
Source: King’s Centre for Visualization in Science, at https://applets.kcvs.ca/IRWindows/IRWindows.html
Carbon dioxide absorbs strongly in two narrow ranges, each resulting from excitation of particular modes of vibration (See Module 2709 Why are nitrogen and oxygen not greenhouse gases?)
And it so happens that the two absorption bands of carbon dioxide are at wavelengths that water vapour does not absorb.
We say that carbon dioxide absorbs radiations in the “transparency window” of water vapour.
And it so happens that the two absorption bands of carbon dioxide are at wavelengths that water vapour does not absorb.
We say that carbon dioxide absorbs radiations in the “transparency window” of water vapour.
And since they are both in the atmosphere?
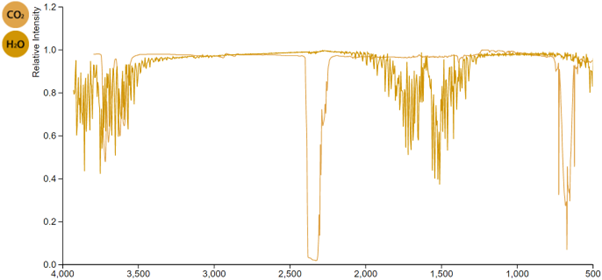
Here are the two absorption spectra superimposed.
Source: King’s Centre for Visualization in Science, at https://applets.kcvs.ca/IRWindows/IRWindows.html
This overlay clearly shows that water vapour absorbs infrared radiations emitted from the surface of Earth in two wavelength ranges – one near 3700 nm, and another near 1600 nm. Carbon dioxide specifically absorbs radiations around 2400 nm, and around 700 nm.
In summary, carbon dioxide absorbs the same radiations with the same wavelengths that it would if there were no carbon dioxide in the atmosphere.
In summary, carbon dioxide absorbs the same radiations with the same wavelengths that it would if there were no carbon dioxide in the atmosphere.
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules in each chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.