Chapter 11-specific PCK
PCK1103
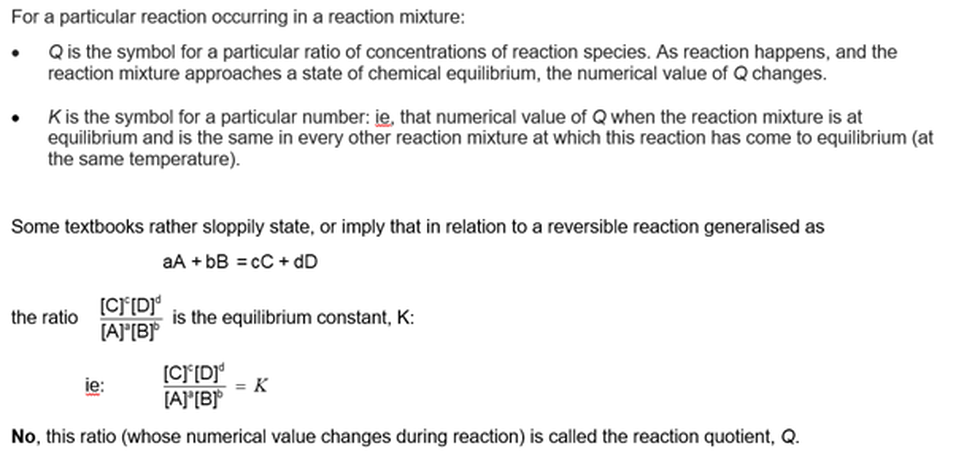
Equilibrium constants: The law of equilibrium
With reference to Learning module 1103 Equilibrium constants: the law of equilibrium
What is the real meaning of an equilibrium constant?
Language issues (again)
The law of equilibrium is often expressed in textbooks, and understood by students, in such a way that it implies that when a reaction mixture is at equilibrium, the function of concentrations that we call the reaction quotient, Q, has a constant value over time.
This is correct, but every imaginable function of concentrations of reactant species is constant with time in a reaction mixture at equilibrium – for the simple reason that the concentrations of all of the species are constant.
The real meaning of the law of equilbrium is much more profound that this.
What is so special about that function of concentrations that we call the reaction quotient, Q? Well, if we have many reaction vessels in which a give reaction is at equilibrium, then Q has the same value in all of them. This deeper meaning is amazing – and is not the case for any other function of concentrations.
I suggest that the shallower meaning expressed in the first paragraph is due to the way that the law of equilibrium is often stated. As is so often the case, it is all a matter of language!
The law of chemical equilibrium is often stated something like this: In a system at equilibrium, at a specified temperature, the function Q (defined) is a constant.
The words in bold are language traps for novices.
To chemists, the word system refers to a type of reaction. For example, chemists might talk about the NO2/N2O4 system – referring to all reaction vessels in which there is the reaction represented by the equation 2 NO2(g) = N2O4(g)
However, to most students, it is probable that the words “a system” induce visualization of one reaction vessel.
And the word constant? What does this word mean in everyday life – and presumably to most students? Yes that’s correct: it means ….. not changing as time passes.
Scientists, however, sometimes use the word “constant” to mean the same in all cases.
So, I think there is value in expressing the law of equilibrium in a way that deals with both of these language issues in such a way as to state clearly the true meaning – by referring to multiple reaction mixtures, and to avoid using the word “constant” for the value of Q ….. as follows:
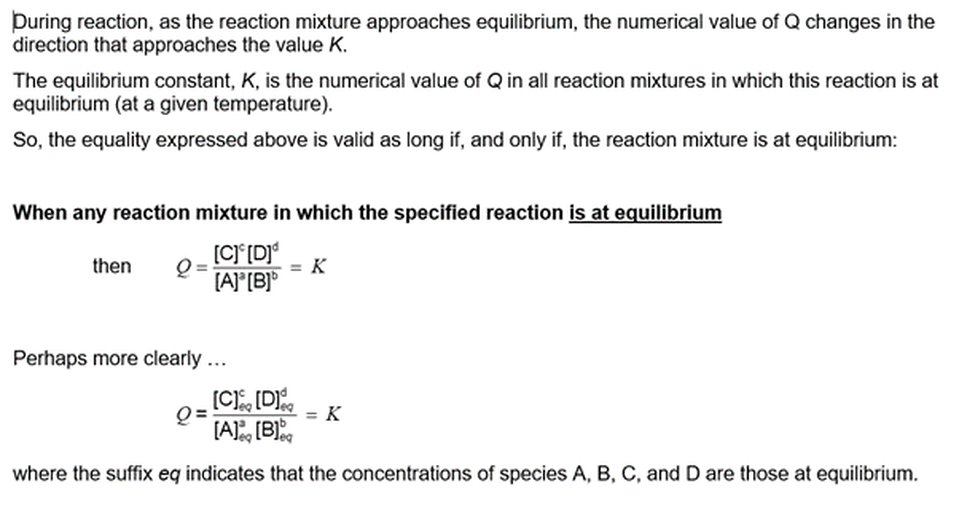
In all reaction mixtures in which a particular reaction is at equilibrium, and at the same temperature, the reaction quotient Q has the same numerical value. We call this numerical value the equilibrium constant for that reaction at that temperature.
[Where of course, Q, the function of species concentrations, needs to be defined in relation to the way that the chemical equation is written]
The law of equilibrium is often expressed in textbooks, and understood by students, in such a way that it implies that when a reaction mixture is at equilibrium, the function of concentrations that we call the reaction quotient, Q, has a constant value over time.
This is correct, but every imaginable function of concentrations of reactant species is constant with time in a reaction mixture at equilibrium – for the simple reason that the concentrations of all of the species are constant.
The real meaning of the law of equilbrium is much more profound that this.
What is so special about that function of concentrations that we call the reaction quotient, Q? Well, if we have many reaction vessels in which a give reaction is at equilibrium, then Q has the same value in all of them. This deeper meaning is amazing – and is not the case for any other function of concentrations.
I suggest that the shallower meaning expressed in the first paragraph is due to the way that the law of equilibrium is often stated. As is so often the case, it is all a matter of language!
The law of chemical equilibrium is often stated something like this: In a system at equilibrium, at a specified temperature, the function Q (defined) is a constant.
The words in bold are language traps for novices.
To chemists, the word system refers to a type of reaction. For example, chemists might talk about the NO2/N2O4 system – referring to all reaction vessels in which there is the reaction represented by the equation 2 NO2(g) = N2O4(g)
However, to most students, it is probable that the words “a system” induce visualization of one reaction vessel.
And the word constant? What does this word mean in everyday life – and presumably to most students? Yes that’s correct: it means ….. not changing as time passes.
Scientists, however, sometimes use the word “constant” to mean the same in all cases.
So, I think there is value in expressing the law of equilibrium in a way that deals with both of these language issues in such a way as to state clearly the true meaning – by referring to multiple reaction mixtures, and to avoid using the word “constant” for the value of Q ….. as follows:
In all reaction mixtures in which a particular reaction is at equilibrium, and at the same temperature, the reaction quotient Q has the same numerical value. We call this numerical value the equilibrium constant for that reaction at that temperature.
[Where of course, Q, the function of species concentrations, needs to be defined in relation to the way that the chemical equation is written]
Clarifying the science content
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.