Module 2201
Quantisation of forms of energy
Why do 'excited' hydrogen atoms emit radiation of only particular energies? How do we explain line emission spectra?
Why in the visible and ultraviolet parts of the spectrum?
Electrons in atoms can only have particular amounts of energy!?
Why are absorption or emission spectra due to change of quantised vibrational levels in the infrared region?
Why are spectra due to change of energy levels of rotation of molecules in the microwave region?
Tune in to Prof Bob .......
Wondrous stuff, you have to agree? Is your own personal energy quantised?
KEY IDEAS - Quantisation of forms of energy
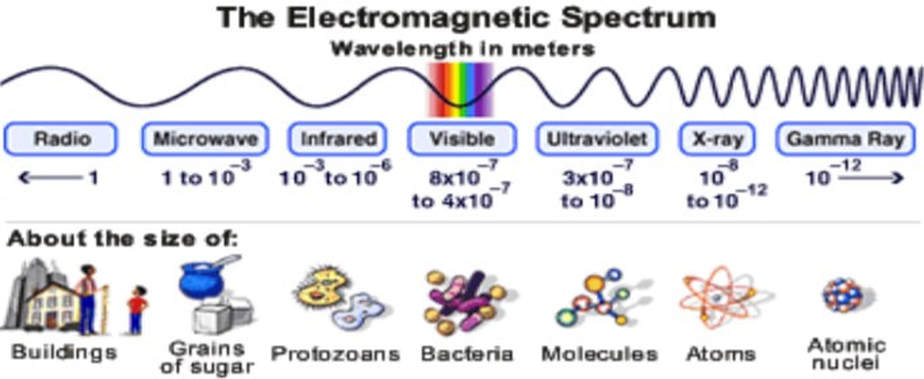
The electromagnetic spectrum: The full range of wavelengths of radiation
I refer to various regions of the electromagnetic spectrum, so let’s understand what these various regions are.
Source: NOAA (National Oceanic and Atmospheric Administration) https://www.esrl.noaa.gov/gmd/outreach/info_activities/pdfs/TBI_electromagnetic_radiation.pdf
Waves range from the long-wavelength (low-frequency) radio waves to the short-wavelength (high-frequency) gamma rays. An alternative conception of radiation is as particles, or “quanta”. The quanta corresponding with radio waves are low-energy particles, and those corresponding with gamma rays have high energy.
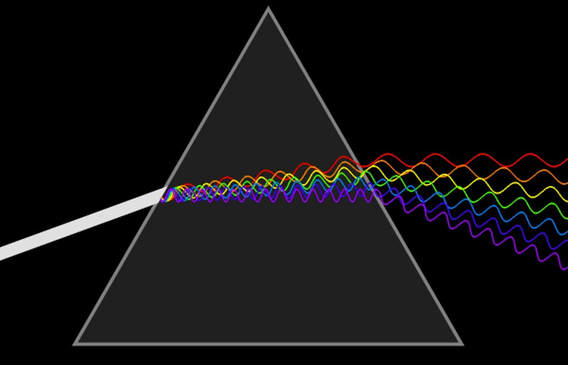
'White light'
“White light” can be shown (by diffraction through a prism) to consist of all of the colours of the rainbow; that is, all of the wavelengths (or frequencies, or quantum energies) of radiation that we see as colours.
Image credit: https://www.weather.gov/jetstream/color
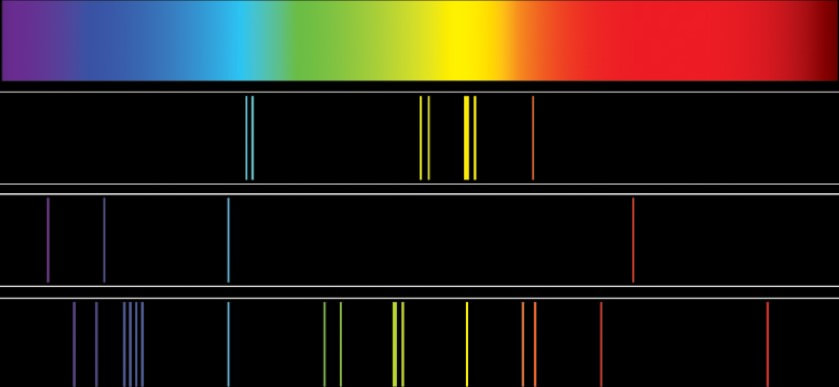
The evidence: Line emission spectrum of 'excited' atoms at visible and ultraviolet frequencies
In contrast to white light, the radiation emitted by a sample consisting of hydrogen atoms (at high temperature, or subjected to a high electrode potential) has only particular wavelengths: there are no radiations emitted with wavelengths other than these.
The emission spectrum from other substances existing as atoms, and that have been “excited” by heat or electric potential, are similar to that of atomic hydrogen, although the particular wavelengths emitted are different. Hydrogen atoms have only one electron: all other atoms have more than one.
How to explain? Can you imagine the arousal of curiosity among chemists of the day!?
An explanatory model: quantisation of energy
Niels Bohr provided a level of explanation that involved an idea that is as extraordinary now as it was then: that the electron in each hydrogen atom can only have particular energies. This is called quantisation of the electron’s energy.
Bohr’s proposition, now commonly accepted was that emission spectra arise from the energy of the electron falling from one “allowed” energy level to a lower one. A quantum of radiation is emitted whose energy is that of the difference between the upper and lower “allowed levels.
This energy of the emitted quantum (which can be regarded as a waveform of particular frequency and wavelength) is one of the lines detected in the spectrum.
A number of “de-excitations” are possible (such as level 2 down to level 1, level 3 to level 1, level 3 to level 2, …….), and for each one, a quantum of particular energy, equal to the difference between the energies of the upper and lower levels) is emitted. Each gives rise to a line in the emission spectrum.
It happens that the differences between the energy levels of electrons are such that the energy of the emitted quanta correspond with the visible region (those which our eyes can detect) and more energetic (ultraviolet radiation).
Absorption spectra
Absorption spectra are the opposite of emission spectra. When “white light” is passed through atomic substances, radiations of only certain wavelengths are absorbed.
These absorption spectra are entirely consistent with the notion of quantisation of the energies of electrons: the electrons can only be “excited” from one “allowed” energy level to another “allowed” energy level. And the energy difference of the jumps corresponds exactly with the energies of the quanta absorbed to allow the jump to happen.
These absorption spectra are entirely consistent with the notion of quantisation of the energies of electrons: the electrons can only be “excited” from one “allowed” energy level to another “allowed” energy level. And the energy difference of the jumps corresponds exactly with the energies of the quanta absorbed to allow the jump to happen.
Spectroscopy in the infrared range: vibrational energy quantised
Molecular substances also absorb particular wavelengths of radiation in the infrared range. Atomic substances do not absorb these radiations of longer wavelength (or lower frequency, or lesser quantum energy) than the visible range. How can this phenomenon be explained?
As well as the energy of the electrons, molecules have a form of energy that atoms cannot have: energy of vibrations. And the energy of each vibrational mode of a molecule (such as stretching, or bending) is quantised: that is, only certain energy levels are possible (we say “allowed”).
So the only energies that are absorbed from radiations passed through a molecular substance are those that correspond with the energy differences between levels.
“Allowed” energy levels of vibration of molecules are closer than the “allowed” energy levels of electrons in atoms, so the energies of quanta of radiation absorbed due to vibrational “excitation” are smaller than those due to electronic excitation.
It happens that the gaps between vibrational energy levels are such that the absorbed quanta have energies in the infrared region.
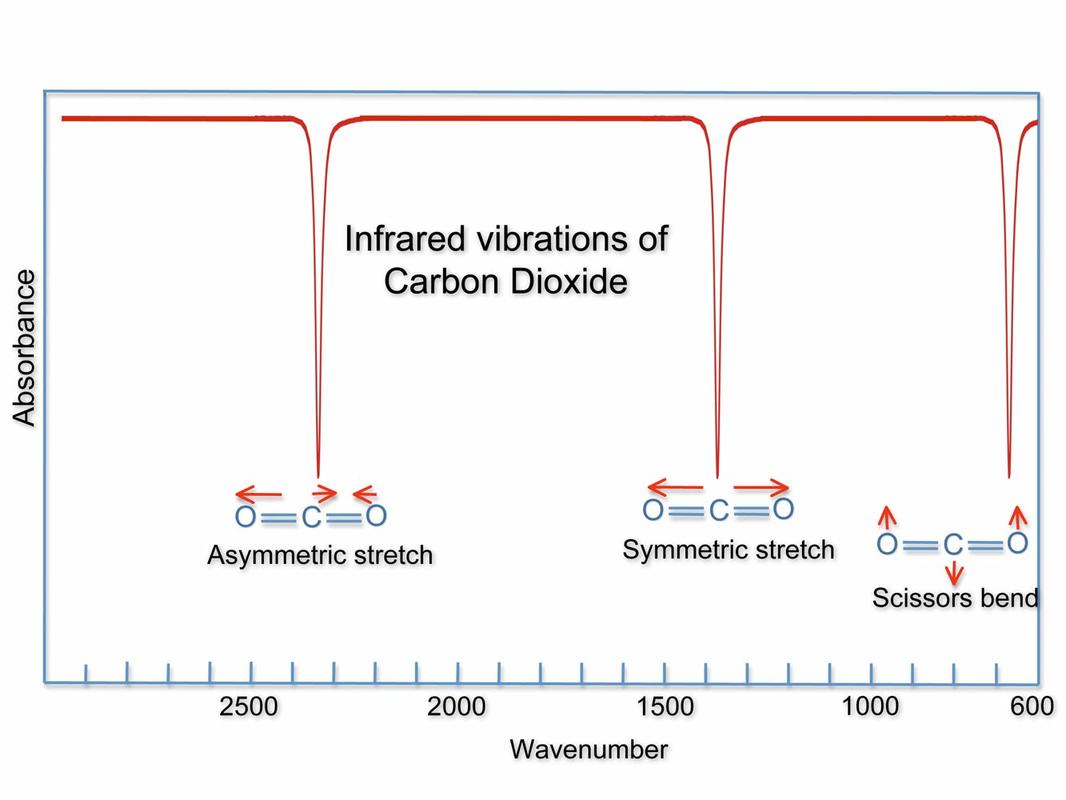
Absorption spectrum of carbon dioxide in the infrared range, showing the specific wavelengths at which radiation is absorbed. The various modes of quantised vibrational levels responsible for the various ranges of absorption are indicated. Wavenumber is a measure of wave frequency: the higher the number, the lower is the frequency.
Source: NOAA (National Oceanic and Atmospheric Administration)
We see bands of absorption, rather than lines, because there may be several modes of vibration (symmetric stretching, antisymmetic stretching, bending) and for each one, there may be excitations between “allowed” vibrational energy levels of different electronic energy levels.
Analysis of the absorption spectra of molecular compounds allows chemists to deduce much information about the structures of their molecules – particularly to allow immediate recognition of the presence of specific functional groups (such as the alcohol –OH group, the carboxylic acid –COOH group, or the aldehyde –CHO group).
The particular ranges of infrared radiation that carbon dioxide absorbs is the reason that it is an important “greenhouse gas” affecting global climate.
Analysis of the absorption spectra of molecular compounds allows chemists to deduce much information about the structures of their molecules – particularly to allow immediate recognition of the presence of specific functional groups (such as the alcohol –OH group, the carboxylic acid –COOH group, or the aldehyde –CHO group).
The particular ranges of infrared radiation that carbon dioxide absorbs is the reason that it is an important “greenhouse gas” affecting global climate.
Spectroscopy in the microwave range: quantised rotational energies
Molecular substances also absorb radiations in the long-wavelength (low frequency, low quantum energy) region of the spectrum known as the microwave region. This is attributed to quantization of the energy of rotation (tumbling action) of the molecules.
“Allowed” rotational energy levels are even closer than vibrational energy levels. So the differences between levels are smaller, and the energies of absorbed quanta (equal to the differences between energy levels) are smaller. The corresponding wavelengths of the radiations are in the microwave region.
Microwave absorption spectra have been a valuable source information about the structure of molecules.
SELF CHECK - Some thinking tasks
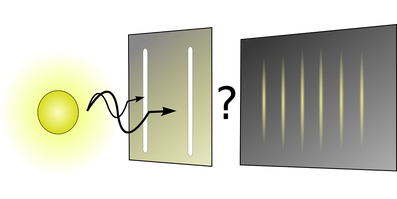
Radiation from an excited hydrogen atom that is passed through the slits of a diffraction grating can be seen to have only particular energies. The particular emitted energies do NOT correspond the 'allowed' energy levels of the electron in hydrogen atoms. Rather, they correspond with the GAPS between the energy levels. Got it? Test yourself.
Answers
1. (i) Quanta emitted can have 2, 3, 4, 5, 7, or 9 units of energy.
(ii) Quanta absorbed can have 2, 3, 4, 5, 7, or 9 units of energy.
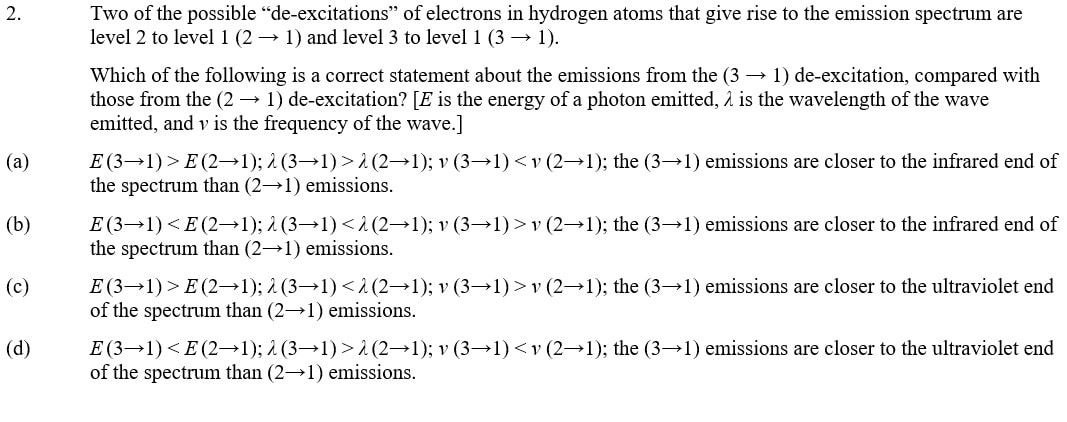
2. (c)
3. (d)
4. (a)
1. (i) Quanta emitted can have 2, 3, 4, 5, 7, or 9 units of energy.
(ii) Quanta absorbed can have 2, 3, 4, 5, 7, or 9 units of energy.
2. (c)
3. (d)
4. (a)
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.