Module 0501
Chemical amount, and its unit of measurement - mole
What is the meaning of chemical amount?
How do chemists use it?
Counting by weighing?
How do chemists measure the chemical amount of stuff?
What is the unit of measurement of chemical amount?
Chemical amount: Preamble
One of the most challenging concepts to understand in learning chemistry for understanding is that of chemical amount.
Prof Bob and some others use the term chemical amount instead of the official term amount of substance (often abbreviated to simply amount) - but the latter sounds too general, and does not distinguish it's specific meaning from other everyday measures of amount - such as mass, or volume, or number.
The usefulness of the concept of chemical amount is that it allows us to "count" the number of atoms or molecules in a sample of substance by weighing the sample. The link between its mass and the number of particles is the chemical amount, of which the unit of measurement is a mole.
Chemical amount of what? Well, we can talk about the chemical amount of a substance in a sample of it, or the chemical amount of a particular species (atom, molecule, or ion) in a sample of stuff.
Quantities, and their units of measurement
It can be a bit tricky trying to make sense of an abstract concept like chemical amount, and a seemingly equally abstract unit of measurement. But you are looking to learn chemistry for understanding, aren't you?
In fact, the unit of measurement of any quantity is an equally arbitrary human construct. For comparison, let's begin by looking at some other quantities that we use every day, and their units of measurement. [Quantity is the general term used to refer length, volume, mass, chemical amount, etc.]
- The quantity called length is measured in a unit called metre. If objects A and B have the same number of metres, they are said to be of equal length.
- The quantity called volume is measured in a unit called litre. If objects A and B have the same number of litres, they are said to be of equal volume.
- The quantity called chemical amount is measured in a unit called mole. If samples A and B have the same number of moles, they are said to have the same chemical amount.
But we haven't yet got around to making sense of what is meant when we refer to the quantity chemical amount. It is a little curious that the easiest way to define the concept of chemical amount is by reference to its unit of measurement (mole). Over to Prof Bob .........
Prof Bob and Aussie explore the meanings of the terms "chemical amount" and "mole".
KEY IDEAS - Chemical amount, and the mole
'Counting' by weighing - an analogy
If you take a bag of $5 coins to the bank, do they count them out? No, they weigh them. Knowing the weight of each coin, they can easily calculate the number of coins. Knowing the worth of each coin, it is easy to calculate the total value.
So it is with estimating the number of particles in a sample of substance. If we define some 'packet' to have a specified number of particles, and we know the mass of each packet, then the total mass of the sample tells us how many of those particles are in the sample.
In the banking analogy above, the 'packet' is a $5 coin and it defines a value of five units of money. If each coin has a mass of 0.250 g, and the mass of your bag of coins is 150 g, the bank teller will say that you have the equivalent of 600 units of money - ie, 600 lots of $1 coins.
In chemistry, the basic 'packet' is called a mole. And each packet corresponds with a lot more than 5 units, where each unit is one particle of the substance ... as we shall see.
Defining chemical amount (via the mole)
One mole of a substance is the amount of it that contains 6.02214076 × 10^23 particles of specified type.
That is: 602214076000000000000000 particles
... usually rounded off to four significant figures as 6.022 x 10^23 particles
The chemical amount of a species is a measure of the number of that species in a particular sample. It is defined as follows ...
The chemical amount of a sample of a substance is 1 mol if that sample contains 6.02214076 × 10^23 particles of specified type.
It might be clarifying to realise that this definition corresponds with the following definitions of other quantities in terms of their units of measurement:
- The length of an object is one metre if it has the same length as [the way the metre is currently defined. See metre]
- The mass of an object is one kilogram if it has the same mass as [the way the kilogram is currently defined. See kilogram]
So, a bag containing 10 parcels (oops ... moles) of a substance has in it 6.022 x 10^24 particles.
And a bag containing 0.10 of a mole of a substance has in it 6.022 x 10^22 particles.
And if we know the mass of one mole of the substance (which is different from substance to substance), we can 'count' the particles by weighing the sample of substance.
All we need to know is the mass of a mole of each substance. How can we know that? Read on ........
Notation, symbols, language
The accepted international symbol for chemical amount is n.
If the chemical amount of a species or substance is 0.37 mol, we write n = 0.37 mol
More specifically, if the chemical amount of a sample of water is 0.500 mol (ie, 9.007 g), we write n(H2O) = 0.500 mol
If the chemical amount of a sample of solid sodium chloride is 0.0091 mol, we write n(NaCl) = 0.0091 mol
By the way, it is fairly common for people to write or say, for example: "The number of moles of stuff in the sample is 0.219".
Think about it ... Do we say "The number of metres of the table is 1.65"? No, we say "The length of the table is 1.65 m". The extent of the quantity (length) is expressed as a number of the unit of measurement.
Do yourself a favour in long term and say it this way: "The chemical amount of the stuff is 0.291 mol".
Specified particles?
Usually, the particles specified are those of which the substance is composed:
- In the case of a metal like iron, Fe(s), these are Fe atoms.
- For the molecular substance water, H2O(l), this refers to H2O molecules..
- For an ionic compound like sodium chloride, NaCl(s), we can specify either Na+ ions or Cl- ions.
That number ...... The Avogadro constant
The number 6.022 x 10^23 (more precisely 6.022 140 76 x 10^23) is called the Avogadro constant (symbol NA). See Module 0502 The Avogadro constant; How many is that? and Module 0503 The Avogadro constant: Why that number?
[Technically, the Avogadro constant has units since it refers to the number of particles per mol.
(NA = 6.022 140 76 x 10^23/mol).
The numerical value of the Avogadro constant (6.022 140 76 x 10^23) is called the Avogadro number.]
The masses of 1 mol of substances: molar mass
One mole of each substance (the amount of it that contains 6.022 × 10^23 particles) has a particular mass, – different from substance to substance.
The mass of exactly 1 mol of any substance is called its molar mass (symbol M). For example M(Fe) = 55.85 g/mol, and M(NaCl) = 58.44 g/mol]
The mass of 1 mol of substances can be calculated as follows:
- For a substance composed of atoms (eg; Fe(s), C(s), He(g)): The atomic weight, expressed in grams.
- For a substance composed of molecules (eg; H2O(l), C2H5OH(l), N2(g)): The molecular weight, expressed in grams – that is, the sum of the atomic weights of all of the atoms in each molecule.
- For an ionic substance (eg; NaCl, MgCl2): The sum of the atomic weights of the atoms expressed in its formula.
By definition, the number of particles in 1 mol of any substance is the same (6.022 × 10^23) as the number of particles in 1 mol of any other substance.
It follows that, for example, the number of particles in 0.01 mol of any substance is the same (6.022 × 10^21) as the number of particles in 0.010 mol of any other substance. And 2 mol of any one of these substances contains twice as many particles as in 1 mol of any other. And so on …
Chemical amount: So what?
The concept of chemical amount is the foundation of all quantitative chemistry. When you are learning chemistry (or doing chemistry) you can't get very far without reference to the amount of a substance or species, measured in moles.
Foreshadowing the content of Module 0505 Chemical equations: What can they tell us? and Module 0506 Limiting reactants: How much reaction can happen? if we know the balanced chemical equation for a reaction, we now have the tools to do the following ……
- Usually we don’t need to know the absolute numbers of particles of substances that react or are produced – just the ratio of the numbers (which the reaction equation tells us).
- The ratio of the numbers or particles of the substances is the same as the ratio of the amounts (in moles) of them.
- From the ratio of the amounts (in moles) of the substances, we can use the molar masses to calculate the relative masses that react or are produced.
- If we know the amount (in moles) of any one substance that reacts or is produced, we can know the amount (in moles) of any other substance that reacts or is produced (from the reaction equation), and then its mass (from its molar mass).
And foreshadowing the content of Module 0907 Solution concentration, if the concentration of a solution is known, we can know the chemical amount (and, therefore, the number of particles of a species) in any given volume of the solution.
1 mol of each of (i) nitrogen gas at STP, mass 28.02 g; (ii) iron, mass 55.85 g; (iii) water (with colouring), mass 18.02 g; and (iv) sodium chloride, mass 58.44 g.
How big would a sample of 1 mol of table sugar look alongside these? You might need to calculate its mass to get an idea. Table sugar is sucrose, whose formula is ..... ?
How big would a sample of 1 mol of table sugar look alongside these? You might need to calculate its mass to get an idea. Table sugar is sucrose, whose formula is ..... ?
Footnote: Other information resources
It's probably obvious that chemical amount, mole, and the Avogadro constant are interdependent on one another. You might find it interesting how these are defined by the International Union of Pure and Applied Chemistry (IUPAC), the world governing body of chemistry.
IUPAC has published the Compendium of Chemical Terminology (also called the IUPAC Gold Book) with up-to-date definitions for every term used in the field of Chemistry. The definitions are, of course, strictly accurate, but since they have been formulated by chemists, and for chemists, you might find the mode of expression interesting (and perhaps odd?).
Follow the links to see the definitions of chemical amount (also called amount of substance), and mole.
In Wikipedia you will find broader discussions, with some historical context, of amount of substance and mole.
There are similar sources for the Avogadro constant, which I will cite in the module 0503 The Avogadro constant: Why is it that number?
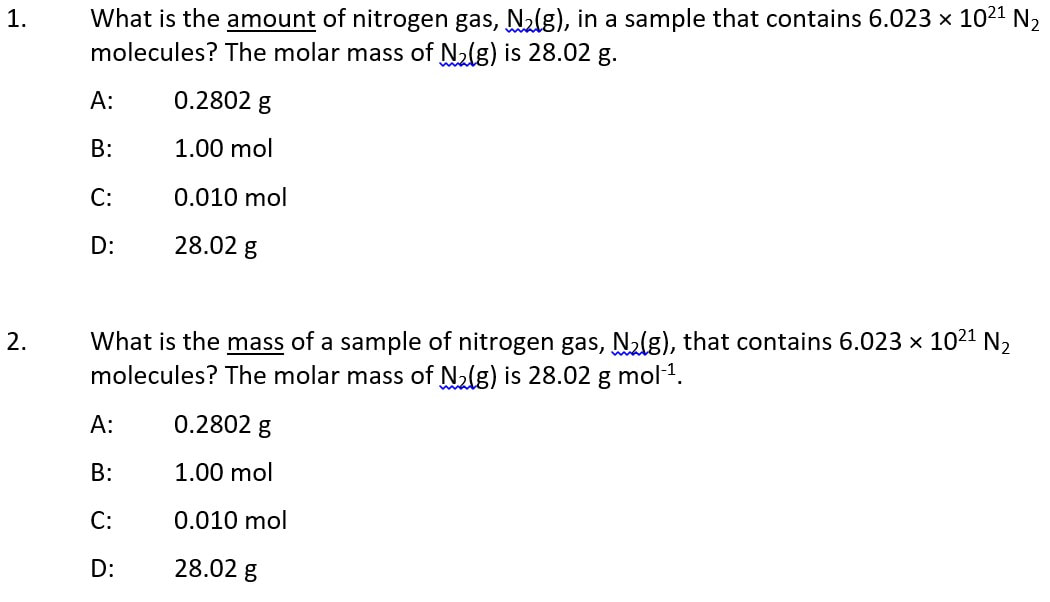
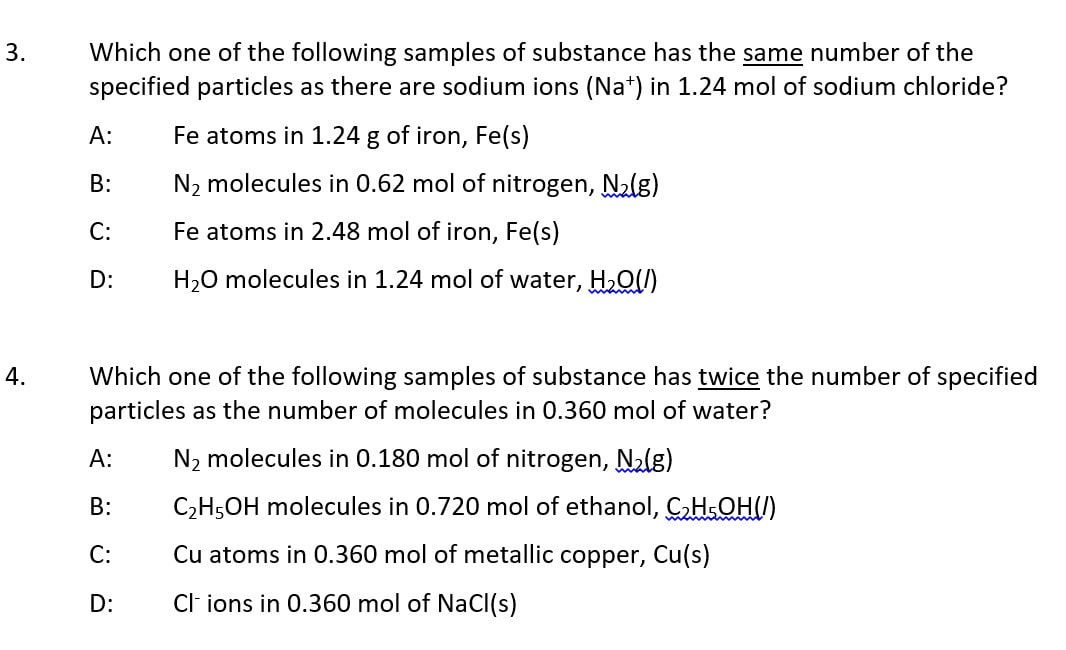
SELF-CHECK: Some thinking tasks
Answers: 1 (C); 2 (A); 3 (D); 4 (B); 5 (C); 6 (A)
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.
Seek wisdom, knowledge, enjoyment, and satisfaction