Module 2706
Why do carbon dioxide molecules absorb radiations emitted from Earth?
Is it because of some characteristic of carbon dioxide molecules?
Or because of some characteristic of the radiations?
Or both?
A match made in heaven (well, not that high up ….)
Before we begin ....
Let’s give some perspective to our questions:
- Many other substances don’t absorb the radiations emitted from the surface of Earth. That includes the major components of air – nitrogen and oxygen (Module 2709 Why are nitrogen and oxygen not greenhouse gases?)
- Carbon dioxide does not absorb some other radiations – for example the visible light that comes to Earth from the sun (Module 2708 Why doesn’t carbon dioxide absorb the radiation from the sun?).
So it seems that carbon dioxide molecules and the particular radiations emitted from Earth are made for each other. There is something special going on between them.
Here is Prof. Bob's explanation .....
And an approximate transcript of what Prof Bob said ......
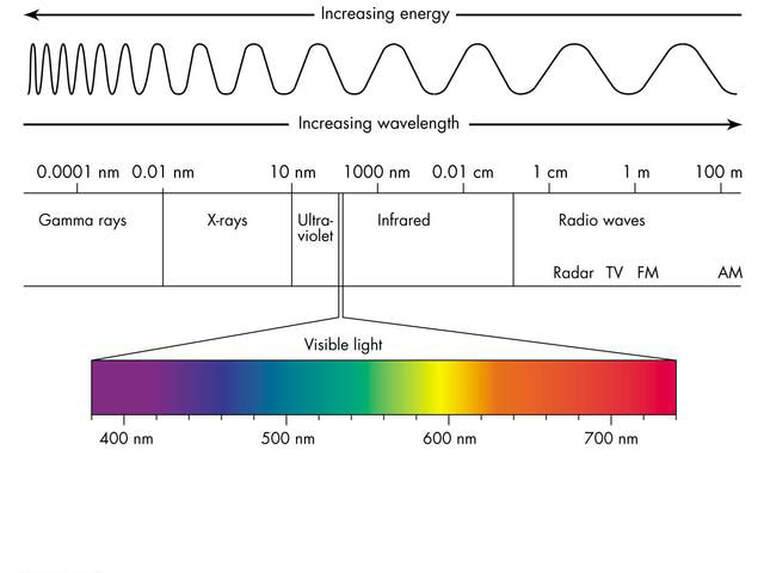
What is characteristic about the emitted radiations?
The wavelengths (or photon energies) of the radiations emitted from Earth are dependent only on the temperature of the Earth’s surface (See Module 2708 Why doesn’t carbon dioxide absorb the radiation that comes from the sun?).
These radiations are in the “longwave infrared” regions of the electromagnetic spectrum.
These radiations are in the “longwave infrared” regions of the electromagnetic spectrum.
So what? you might ask. Be patient.
Let's look at the other side of the equation .....
Carbon dioxide molecules: How "excited" can they get?
I’m not sure if is more technically correct to say that a molecule absorbs a photon, or if it absorbs the energy of the photon. Let’s not get too hung up about it: either way, the photon no longer exists, and the molecule has more energy than before.
What follows is enough for you to get the hang of what’s going on, but is not the full detailed story. Is it ever?
When a carbon dioxide molecule absorbs a photon of radiation emitted from the surface of Earth, the increased energy of the molecule results in faster vibrations of the atoms.
Keep in mind that the question of this module is why the molecule absorbs the photon.
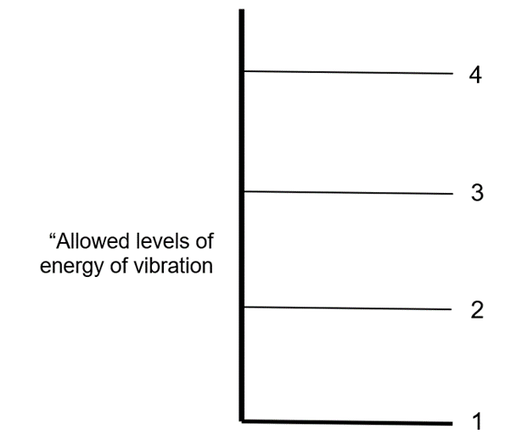
The answer is ….. “Quantization” of the energy of vibration of molecules: Molecules can only vibrate at particular levels of energy. They cannot vibrate with energy different from those “allowed” levels.
You may have encountered the notion of quantisation previously in another context (Module 2201 Quantisation of forms of energy).
The answer is ….. “Quantization” of the energy of vibration of molecules: Molecules can only vibrate at particular levels of energy. They cannot vibrate with energy different from those “allowed” levels.
You may have encountered the notion of quantisation previously in another context (Module 2201 Quantisation of forms of energy).
So, a molecule can absorb a photon only if the energy of the photon is the same as the difference between “allowed” levels. Otherwise the energy would increase to a level that is not “allowed”.
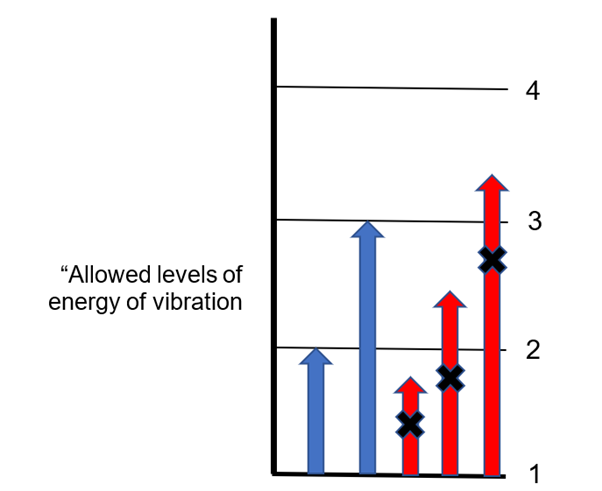
Jumps in energy represented by the blue arrows (from level 1 exactly to level 2 or level 3) are possible. But jumps represented by the red arrows would take the energy of vibration to a level that is not “allowed”. So, the molecule can absorb a photon whose energy is the same as that portrayed by a blue arrow, but cannot absorb photons with energies different from these.
Here’s the rub. Putting one and one together ….
It just happens that the energies of photons of the longwave infrared radiations emitted from the surface of Earth are closely equal to the gap between levels 1 and 2 of the vibrational energy of carbon dioxide molecules.
So they can be – and are – absorbed.
If the emitted radiations had longer or shorter wavelengths, the carbon dioxide molecules would usher the photons on their way to space, with a polite "Sorry, I can't take you." That would be called transmission (rather than absorption) of the radiation.
So they can be – and are – absorbed.
If the emitted radiations had longer or shorter wavelengths, the carbon dioxide molecules would usher the photons on their way to space, with a polite "Sorry, I can't take you." That would be called transmission (rather than absorption) of the radiation.
What a match – carbon dioxide molecules and longwave infrared photons! Coincidence, evolution, or Mother Nature?
Infrared absorption spectra
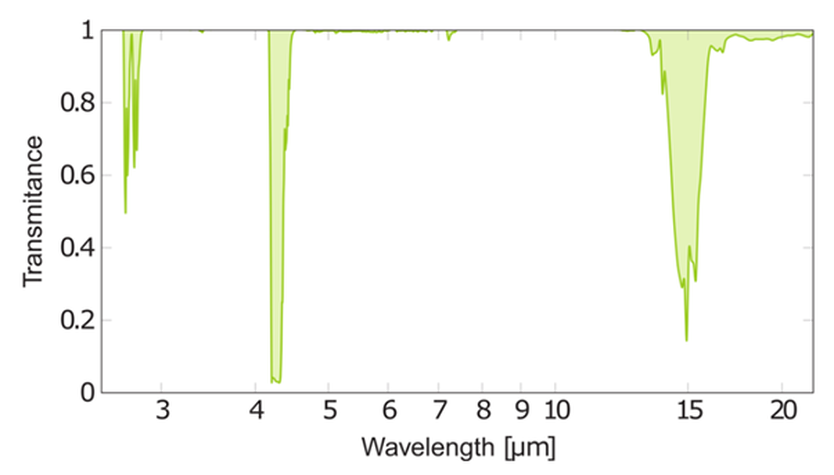
Here is a trace of how much of infrared radiation is absorbed (or transmitted) by carbon dioxide as we gradually change the wavelength of the radiation.
The spectrum shows us that carbon dioxide absorbs radiation with wavelength about 4.3 µm (4300 nm). The energy of these photons is equal to the gap between “allowed” energy levels of the molecules.
Radiations with wavelength 3.5 µm (3500 nm) or 7 µm (7000 nm), for example, are not absorbed: they are transmitted.
My fudge ....
I confess to a simplification of the story to avoid complexity and discombobulation - but which might have had you raising your eyebrows questioningly.
According to my story, the only photons that would be absorbed are those whose energy is exactly equal to the gap between vibrational energy levels 1 and 2 of carbon dioxide molecules. This would result in absorption at exactly one wavelength, rather than over a narrow range of wavelengths, which we call an absorption "band".
In fact, the energy of rotation (or tumbling) of carbon dioxide molecules is also quantised - but the energy gaps are much smaller than for vibrational energy. For every vibrational energy level, there are a number of closely spaced rotational energy levels. Let's label them R0, R1, R2, and so on.
So from vibrational level V1 to vibrational level V2, allowed jumps include V1/R0 to V2/R4, or V1/R3 to V2/R2, and so on. So there are a range of "allowed" energy transitions of approximately, but not exactly, equal size.
According to my story, the only photons that would be absorbed are those whose energy is exactly equal to the gap between vibrational energy levels 1 and 2 of carbon dioxide molecules. This would result in absorption at exactly one wavelength, rather than over a narrow range of wavelengths, which we call an absorption "band".
In fact, the energy of rotation (or tumbling) of carbon dioxide molecules is also quantised - but the energy gaps are much smaller than for vibrational energy. For every vibrational energy level, there are a number of closely spaced rotational energy levels. Let's label them R0, R1, R2, and so on.
So from vibrational level V1 to vibrational level V2, allowed jumps include V1/R0 to V2/R4, or V1/R3 to V2/R2, and so on. So there are a range of "allowed" energy transitions of approximately, but not exactly, equal size.
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.