Module 2709
Why are nitrogen and oxygen not greenhouse gases?
The majority of molecules in the atmosphere are nitrogen and oxygen molecules. Why do they not absorb radiation emitted from Earth?
Carbon dioxide is infrared-active. Why are not nitrogen and oxygen?
Why is carbon dioxide infrared-active?
I can describe to you why. But it is beyond me to explain why, in any sensible way.
Prelude
The essence of the issue is related to the dipole moment of molecules of substances - that is, to their molecular dipole moment (to distinguish from bond dipole moments). The molecular dipole moment is a consequence of charge separations in the molecule due to different electronegativities of the various atoms joined by covalent bonds.
The numerical value assigned to the dipole moments of a molecule is a measure of the tendency of an electrostatic field to turn the molecule in a direction that would align the molecular dipole moment with the direction of the electric field.
The magnitude of the molecular dipole moment is larger
- the larger are the charges on the atoms
- the further apart are the atoms with non-zero charges
- the less the effect of the individual bond dipoles in the molecule cancel out each other.
In principle ...
All molecules vibrate: They rapidly stretch and bend. [In fact, vibration of molecules is actually movement of the atoms of the molecule.]
During vibrations of the molecules of some substances, their dipole moment fluctuates: it changes rapidly, in sync with the vibrations. Such molecules can be “excited” by absorption of infrared radiation. They are said to be “infrared-active”.
The molecules of some other substances are such that their dipole moment does not change during the vibrations. These cannot be “excited” by absorption of infrared radiation. They are “infrared-inactive”.
Okay, that's the rule, but we need to make sense of it specifically in relation to molecules of nitrogen, oxygen, and carbon dioxide ......
During vibrations of the molecules of some substances, their dipole moment fluctuates: it changes rapidly, in sync with the vibrations. Such molecules can be “excited” by absorption of infrared radiation. They are said to be “infrared-active”.
The molecules of some other substances are such that their dipole moment does not change during the vibrations. These cannot be “excited” by absorption of infrared radiation. They are “infrared-inactive”.
Okay, that's the rule, but we need to make sense of it specifically in relation to molecules of nitrogen, oxygen, and carbon dioxide ......
Dipole moments of molecules in their "rest positions"
An important question, with huge implications for our daily lives is whether water molecules, for example, have a dipole moment - that is whether they are polar molecules.
We usually discuss this question by reference to drawings of water molecules in which the atoms appear to be stationary. In fact, the atoms are always jiggling about their positions - and that is what constitutes the vibrations of the molecule. In an accurate drawing, the apparently stationary atoms are in their average position over an extended period of time.
We usually discuss this question by reference to drawings of water molecules in which the atoms appear to be stationary. In fact, the atoms are always jiggling about their positions - and that is what constitutes the vibrations of the molecule. In an accurate drawing, the apparently stationary atoms are in their average position over an extended period of time.
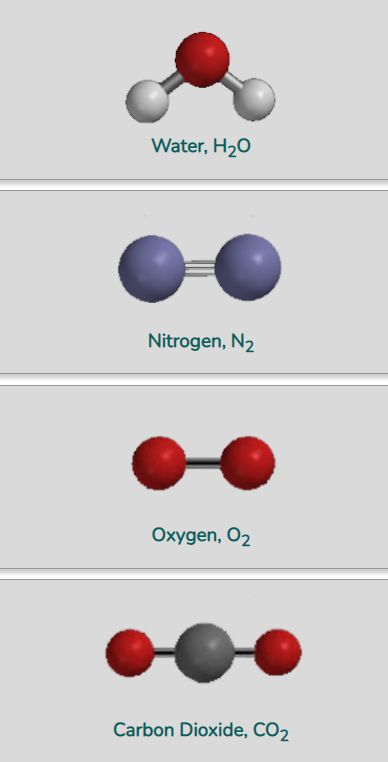
We can deduce from the diagrams above that molecules of nitrogen, oxygen, and carbon dioxide do not have a dipole moment: that is, they are non-polar molecules. On the other hand, water molecules do have a dipole moment.
However, what is important with respect to whether a molecule is infrared-active or not, is not whether the "at rest" molecule has a dipole moment, but whether the dipole moment fluctuates during vibrations of the molecules. Two different things!
Let's see ......
Let's see ......
Nitrogen and oxygen molecules (and other homonuclear diatomic molecules)
The only possible vibration of homonuclear diatomic molecules is stretching: the atoms moving farther apart and then closer together, in rapid repetitive motion. This can be regarded as stretching of the covalent bonds that hold the two atoms together as a molecule.
To see animation of an oxygen molecule vibrating, click here.
If you pause the animation at any time, you can see that, regardless of how the bond is stretched, the molecule has zero dipole moment.
So, the dipole moment does not change during vibrations: it it always zero.
And so, oxygen molecules, as well as nitrogen molecules for the same reason, are not "infrared active". They do not absorb infrared radiation.
And so, they are not greenhouse gases.
So, the dipole moment does not change during vibrations: it it always zero.
And so, oxygen molecules, as well as nitrogen molecules for the same reason, are not "infrared active". They do not absorb infrared radiation.
And so, they are not greenhouse gases.
And carbon dioxide molecules?
Molecules of carbon dioxide, with atoms in their average locations, are non-polar. So how can they be infrared-active?
Well, remember that the criterion is not about whether the molecules have a dipole moment or not, but whether the dipole moment fluctuates in sync with the molecules vibrations.
Well, remember that the criterion is not about whether the molecules have a dipole moment or not, but whether the dipole moment fluctuates in sync with the molecules vibrations.
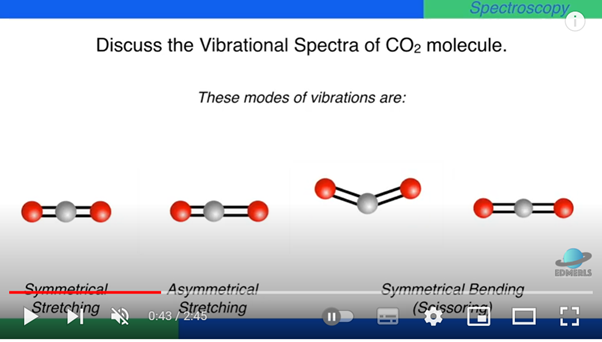
Carbon dioxide molecules can have four different (and simultaneous) ways, called "modes" of vibration:
- Symmetric stretching
- Anti-symmetric stretching
- Bending (or scissoring) - two identical modes, except for the plane of vibration.
Watch the animations of each mode of vibration, pausing at critical frames. You will see:
So, carbon dioxide is infrared-active, and is a greenhouse gas.
- Symmetric stretching: The dipole moment does not change. This mode is not infrared-active. It contributes nothing to carbon dioxide’s absorption of infrared radiation, and action as a greenhouse gas.
- Antisymmetric stretching: The dipole moment does change during vibrations. This mode is infrared-active: it can be “excited” to a higher energy level by absorption of infrared photons.
- Bending: The dipole moment does change during vibrations. These two modes are infrared-active.
So, carbon dioxide is infrared-active, and is a greenhouse gas.
The absorption spectrum of carbon dioxide (for academic interest)
The size of the gap between the "allowed" levels of energy in the two bending modes are equal, but are smaller than the gap between the energy levels of the antisymmetric mode of vibration.
So they absorb photons of different energy (radiations of different wavelength) for energy jumps to the higher "allowed" energy level.
This can be seen in the infrared absorption spectrum of carbon dioxide ....
So they absorb photons of different energy (radiations of different wavelength) for energy jumps to the higher "allowed" energy level.
This can be seen in the infrared absorption spectrum of carbon dioxide ....
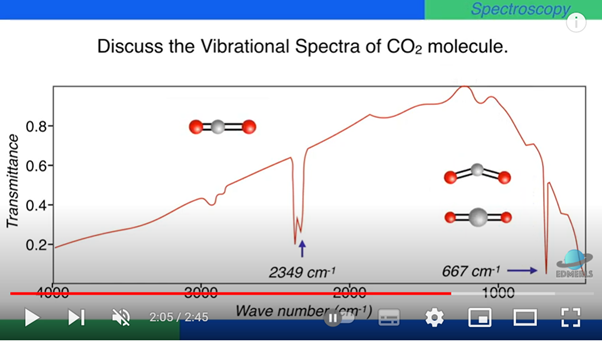
Two strong absorption peaks are due separately to (i) excitation of the asymmetric stretching mode, at 2349 wavenumbers, and (ii) excitation of both bending modes, at 667 wavenumbers. Wavenumber is a unit used by spectroscopists. The higher the wavenumber, the shorter the wavelength of radiation (and the larger the photon energy).
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.