Module 0506
Limiting reactants: How much reaction can happen?
What limits how much reaction can happen?
The amount of one of the reactants
Which one? Why?
Why is the balanced equation important?
Preamble and context
Do reactions go until all of the reactants are 'used up'? Almost never.
Even when the condition of equilibrium is so one-sided in the direction of the products, when one of the reactants has completely reacted, there will still be some of the other(s) remaining unreacted.
What limits how much reaction can happen (when there is no more of one reactant left, so that no further reaction is possible)?
Learning chemistry related to these ideas is limited without sufficient knowledge of the content of at least module 0501 Chemical amount and its unit of measurement, the mole; Module 0505 Chemical equations: What can they tell us? and Module 0507 Balanced chemical reactions; What are they?
Qualifiying note: Closed systems
The discussion in this module about the amount of a reactant that limits further reaction applies to reaction mixtures that are called "closed systems" - that is, happening in a vessel to which none of the reactants or products are added nor removed. This excludes naturally occurring chemical reactions, such as photosynthesis, in which the reactants are continuously being replaced, and the products continuously removed.
Aussie and Prof Bob talk it through .....
Aussie has Aha! moments about which reactant limits the amount of reaction that can happen in a reaction mixture.
KEY IDEAS - Limiting reactants: How much can happen?
Limiting reactant: the one that 'runs out' first
The ability to decide which reactant in a reaction mixture limits the amount of reaction that can happen is dependent on knowing the balanced chemical equation that represents the chemical change.
The amount (in moles) of substances that can react with each other is limited by the amount of that one which is used up first. This is called the limiting reactant.
More reaction can happen if we add more of the limiting reactant. But no more reaction can result from addition of the other reactant – we would just have more of it left unreacted (we say “in excess”).
Stoichiometric calculations (How much of this reacts? How much of that is formed) must be based on the amount (in moles) of the limiting reactant present in the reaction mixture, taking into account the ratio of amounts that react with each other as indicated by the balanced chemical equation for the reaction.
The trick is to understand, given the balanced chemical equation, which reactant will 'run out' first.
The amount (in moles) of substances that can react with each other is limited by the amount of that one which is used up first. This is called the limiting reactant.
More reaction can happen if we add more of the limiting reactant. But no more reaction can result from addition of the other reactant – we would just have more of it left unreacted (we say “in excess”).
Stoichiometric calculations (How much of this reacts? How much of that is formed) must be based on the amount (in moles) of the limiting reactant present in the reaction mixture, taking into account the ratio of amounts that react with each other as indicated by the balanced chemical equation for the reaction.
The trick is to understand, given the balanced chemical equation, which reactant will 'run out' first.
SELF CHECK: Some thinking tasks
ANSWERS
1 (a) E (10)
(b) B (cheese)
(c) D (20)
2 D
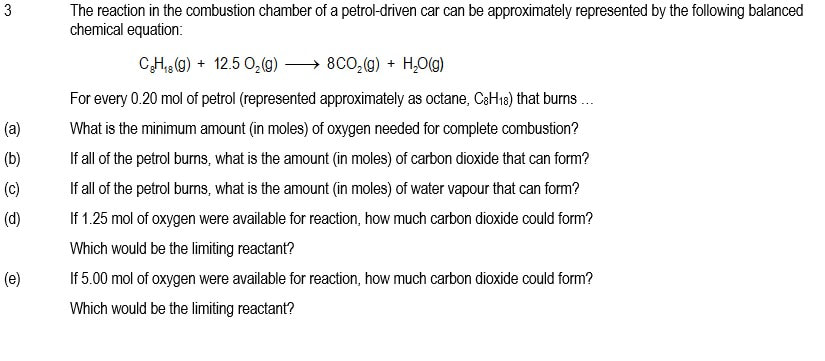
3 (a) 2.5 mol
(b) 1.60 mol
(c) 1,80 mol
(d) 0.80 mol; O2(g)
(e) 1,60 mol; C8H18(g)
(b) B (cheese)
(c) D (20)
2 D
3 (a) 2.5 mol
(b) 1.60 mol
(c) 1,80 mol
(d) 0.80 mol; O2(g)
(e) 1,60 mol; C8H18(g)
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.