Module 2707
The “enhanced greenhouse effect”
How much has the concentration of carbon dioxide in the atmosphere increased since the beginning of the Industrial Revolution?
So what?
In the process of discussing with Aussie the difference between the 'greenhouse effect' and the 'enhanced greenhouse effect', Prof Bob demolishes spurious claims by 'climate deniers',
In the beginning .....
It is 1800 (not quite the beginning). Carbon dioxide has been in our atmosphere forever. So too has been the greenhouse effect (Module 2705 The “greenhouse effect”).
For centuries the concentration of carbon dioxide in the atmosphere has been fairly consistent at about 275 ppm. At this level, the greenhouse effect controls the average temperature at the surface of Earth at about + 15 °C, which is 33 °C higher than if there were no carbon dioxide (Module 2704 Does carbon dioxide affect Earth’s energy balance?).
Carbon dioxide is good!
The greenhouse effect that operated at the concentrations in the atmosphere before the onset of the industrial revolution is sometimes called the natural greenhouse effect.
For centuries the concentration of carbon dioxide in the atmosphere has been fairly consistent at about 275 ppm. At this level, the greenhouse effect controls the average temperature at the surface of Earth at about + 15 °C, which is 33 °C higher than if there were no carbon dioxide (Module 2704 Does carbon dioxide affect Earth’s energy balance?).
Carbon dioxide is good!
The greenhouse effect that operated at the concentrations in the atmosphere before the onset of the industrial revolution is sometimes called the natural greenhouse effect.
The industrial era arrives .....
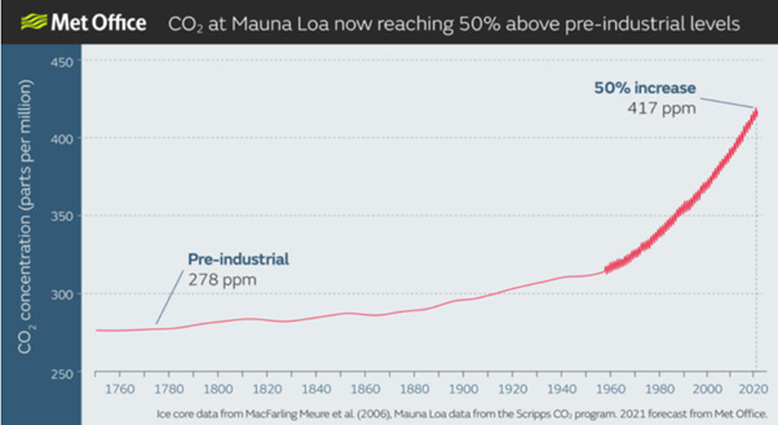
In return for the benefits that we have enjoyed from the industrial era, is there a payoff coming? During this period, the concentration of carbon dioxide in the atmosphere has increased dramatically – although it could still be considered low (42 carbon dioxide molecules in every 100 000 molecules in the air).
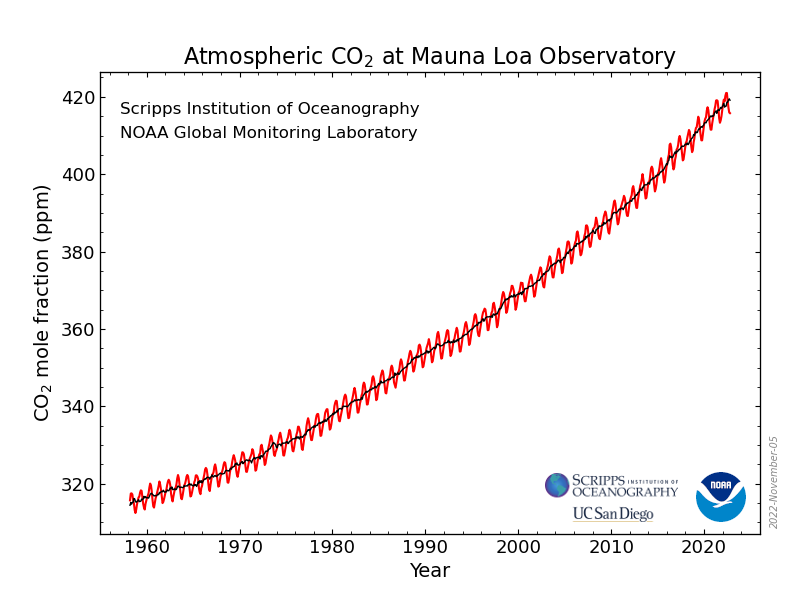
The mean concentration of carbon dioxide in the atmosphere has now reached 421 ppm – a massive 50% increase over the past couple of centuries.
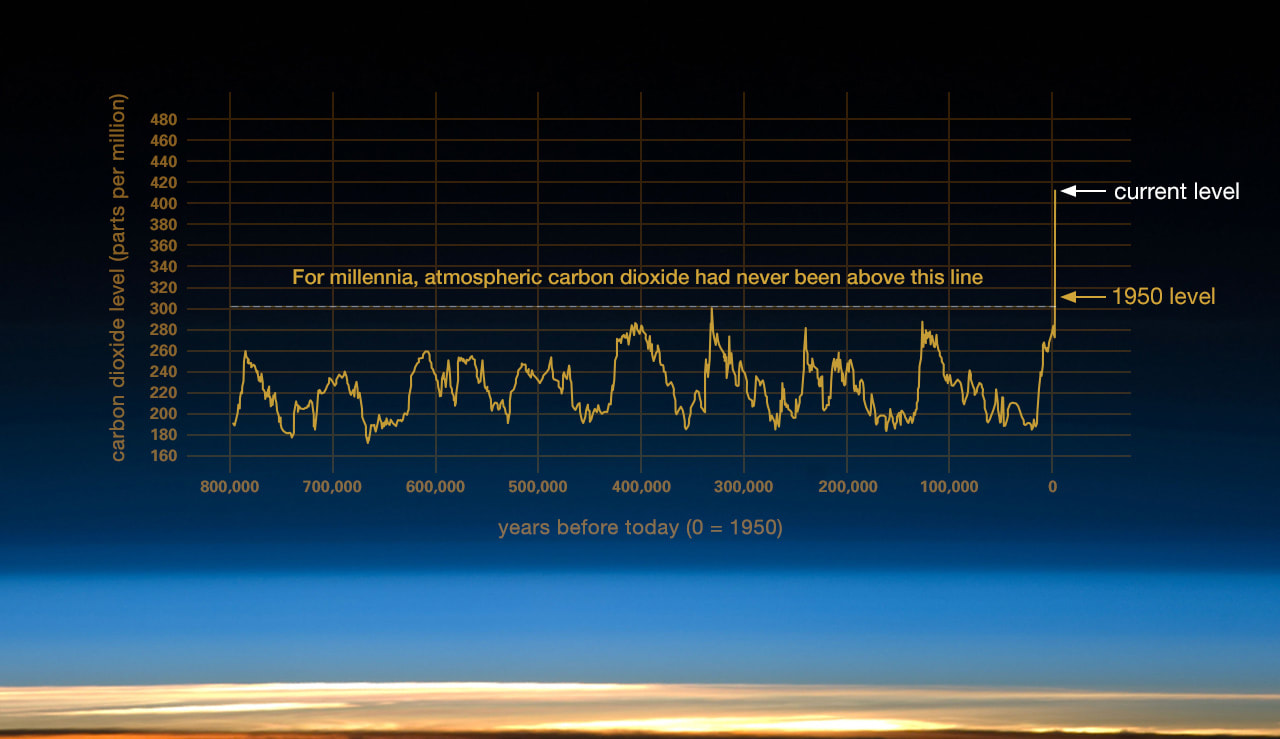
Just natural variation?
Some people say, dismissively .....
They should stop trying to confuse the voters ....
There is no doubt. Ignore the discombobulators.
A link: Carbon dioxide concentration and temperature
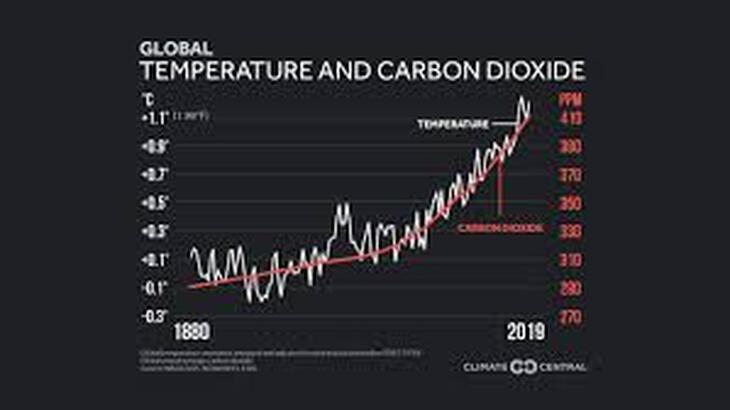
Since carbon dioxide in the atmosphere is responsible for warming of the surface of Earth (the “greenhouse effect”) we should expect that increasing concentrations will lead to still higher temperatures.
Since increasing concentrations of carbon dioxide are enhancing global temperatures, this phenomenon is called the “enhanced greenhouse effect”. This refers to the increase in global temperature due to increasing carbon dioxide concentration since the pre-industrial era.
There has always been the “greenhouse effect” – natural and beneficial to life – with carbon dioxide being the good guy. Now we are experiencing the “enhanced greenhouse effect” – leading to many potential problems facing the human race. Is our perception of carbon dioxide deservedly changing from “good” to “bad”.
Of course, these problems cannot be attributed to carbon dioxide. The fault lies with human beings. Us.
Of course, these problems cannot be attributed to carbon dioxide. The fault lies with human beings. Us.
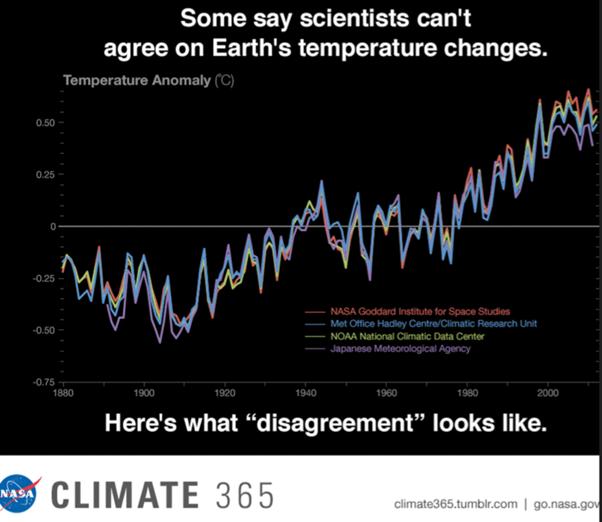
Is the link scientifically proven?
Some people say ....
Yeah well ….
An analogy would be if two different international astronomers have simultaneously, but independently, identified a previously unknown exoplanet. One publishes an estimate that it is 195 742 light years from Earth, but the other has estimated that it is 195 751 light years away.
Do we conclude that they don’t agree, so their findings are untrustworthy – so let’s not believe anything they say?
There is no doubt.
Do we conclude that they don’t agree, so their findings are untrustworthy – so let’s not believe anything they say?
There is no doubt.
And just for interest .....
You may have noticed the wavy values in the concentrations in the graph of increasing carbon dioxide concentrations since direct analyses have been carried out. Let’s have a closer look …..
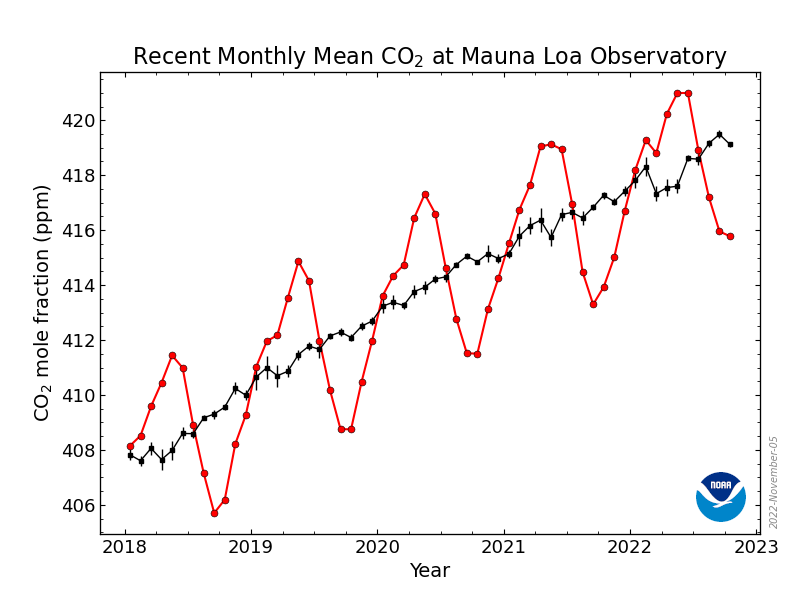
Zooming in further, to the past few years .....
Can you explain that natural phenomenon?
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.