Module 2708
Why doesn’t carbon dioxide absorb the radiation coming from the sun?
Carbon dioxide molecules absorb outgoing radiation from Earth – causing warming?
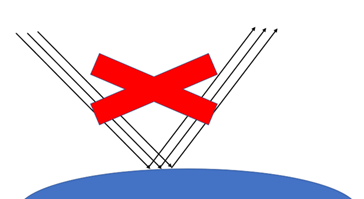
Why, then, don’t they absorb the incoming radiation from the sun – causing cooling?
Why are incoming and outgoing radiations different?
Why? Let's get straight to the heart of the matter ....
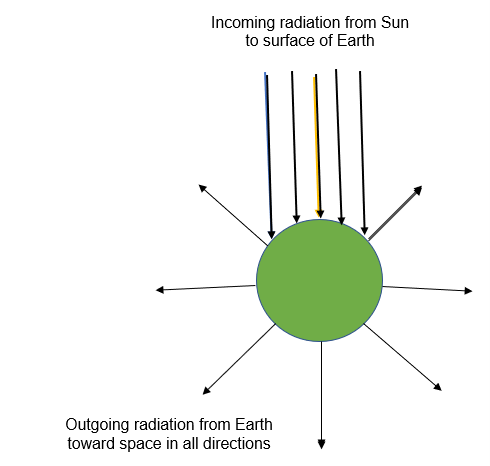
The outgoing radiation from Earth is not the same as the incoming radiation from the sun.
The outgoing radiation has wavelengths in the infrared range, but the incoming radiation from the sun is visible light.
Photons of infrared radiation have energy that matches the gap in the quantised energy levels of vibration of carbon dioxide molecules (Module 2706 Why do carbon dioxide molecules absorb radiations emitted from Earth). So they are absorbed.
Photons of visible light have more energy than photons of infrared light, so absorption by carbon dioxide molecules would take the vibrational energy to a level that is not “allowed”. They cannot be absorbed.
That's it. Simple! But I hear you asking yourself questions ....
The outgoing radiation has wavelengths in the infrared range, but the incoming radiation from the sun is visible light.
Photons of infrared radiation have energy that matches the gap in the quantised energy levels of vibration of carbon dioxide molecules (Module 2706 Why do carbon dioxide molecules absorb radiations emitted from Earth). So they are absorbed.
Photons of visible light have more energy than photons of infrared light, so absorption by carbon dioxide molecules would take the vibrational energy to a level that is not “allowed”. They cannot be absorbed.
That's it. Simple! But I hear you asking yourself questions ....
Why is the outgoing radiation different from the incoming radiation?
First and foremost, the outgoing radiation is NOT (mainly) reflected radiation coming from the sun.
In a state of energy balance, the amount of energy coming in is the same as the amount going out (per unit time), but the photons going out are not the very same photons that came from the sun.
Here is an analogy: I have a glass with water in it. I use a dropper pipette to add one drop of water. Then I use it to take out one drop of water, of exactly the same volume. And I repeat that (adding and withdrawing) many times.
The volume of water in the glass remains constant. The total volume of drops added is equal to the total volume of drops withdrawn.
But the molecules of water that have been withdrawn are not the very same molecules that were added.
Back to Earth's incoming and outgoing radiations, photons of the outgoing radiations are not the very photons that arrived in the incoming radiations. Even more, they do not even have the same energy (or wavelength).
The volume of water in the glass remains constant. The total volume of drops added is equal to the total volume of drops withdrawn.
But the molecules of water that have been withdrawn are not the very same molecules that were added.
Back to Earth's incoming and outgoing radiations, photons of the outgoing radiations are not the very photons that arrived in the incoming radiations. Even more, they do not even have the same energy (or wavelength).
Different sources of incoming and outgoing radiations
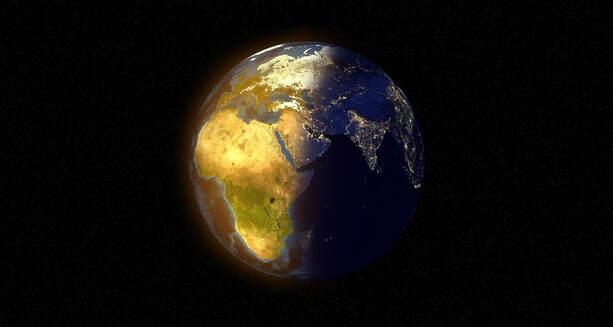
The sun emits radiations in all directions spherically. Earth’s surface receives only that relatively tiny section of the sun’s radiations that come our way.
If outgoing radiations were reflections of the incoming radiations, they would be emitted only from that half of Earth in daylight. That is not the case.
The surface of the Earth absorbs the photons of incoming radiation from the sun, and they immediately cease to exist. But their energy becomes part of Earth's energy.
Photons of outgoing radiation do not exist until the Earth emits them.
The wavelengths of incoming and outgoing radiation, and the energies of their photons, are different because their sources are different.
Photons of outgoing radiation do not exist until the Earth emits them.
The wavelengths of incoming and outgoing radiation, and the energies of their photons, are different because their sources are different.
Earth's incoming radiation is Sun's emitted radiation. So the source of Earth's incoming radiation is the sun The source of Earth's outgoing radiation is ..... Earth.
And the wavelengths are different because the temperature of Earth and Sun are different - a phenomenon known as black-body radiation.
And the wavelengths are different because the temperature of Earth and Sun are different - a phenomenon known as black-body radiation.
Black body radiation
Every object emits radiation whose wavelength (or photon energy) depends only on its temperature - regardless of the material of which the object is composed.
Regardless of composition, every object hotter than about 3000 °C is “white hot”, emitting light ranging across most of the visible spectrum. Near 1000 °C it is “red hot”. From objects in the temperature range 10 – 50 °C, the emitted radiation is invisible to humans: it is infrared radiation.
Regardless of composition, every object hotter than about 3000 °C is “white hot”, emitting light ranging across most of the visible spectrum. Near 1000 °C it is “red hot”. From objects in the temperature range 10 – 50 °C, the emitted radiation is invisible to humans: it is infrared radiation.
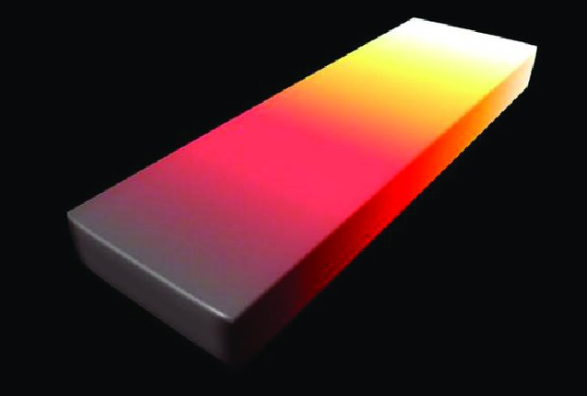
This iron bar, with gradations of temperature, illustrates black-body radiation. At top right, where the temperature is about 3000 °C, the bar is white hot. In the middle, the temperature is about 1000 °C, and it is red hot. If the near end, at about 50 °C were in the dark, we would not see it because it is emitting only infrared radiation.
Outgoing radiation:
The temperature of the surface of Earth is approximately 15 °C. Like all objects at 15 °C, Earth emits radiation whose wavelengths are in the far infrared part of the spectrum.
The photons have energy equal to the gap between the energy levels of vibration of carbon dioxide. So carbon dioxide molecules absorb the photons.
The temperature of the surface of Earth is approximately 15 °C. Like all objects at 15 °C, Earth emits radiation whose wavelengths are in the far infrared part of the spectrum.
The photons have energy equal to the gap between the energy levels of vibration of carbon dioxide. So carbon dioxide molecules absorb the photons.
Incoming radiation
The temperature of the surface of the sun is 5600 °C. Like all objects at such temperatures, the sun emits mainly visible light - “white light” (wavelengths 400 – 700 nm). The energies of the photons (more or less than those of the emitted radiation?) do not match the gap between the energy levels of vibration of carbon dioxide. So carbon dioxide molecules cannot absorb the photons or the energy of the molecules would jump to a level that is not one of the "allowed" ones.
The temperature of the surface of the sun is 5600 °C. Like all objects at such temperatures, the sun emits mainly visible light - “white light” (wavelengths 400 – 700 nm). The energies of the photons (more or less than those of the emitted radiation?) do not match the gap between the energy levels of vibration of carbon dioxide. So carbon dioxide molecules cannot absorb the photons or the energy of the molecules would jump to a level that is not one of the "allowed" ones.
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.