|
Module 0911
Concentrated, dilute, strong, weakDoes the word concentrated refer to solutions, or to solutes?
|
|
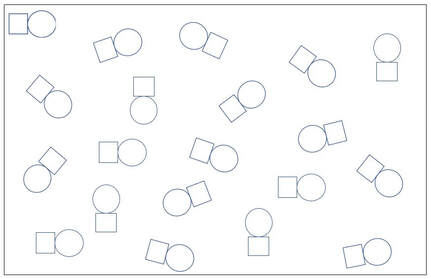
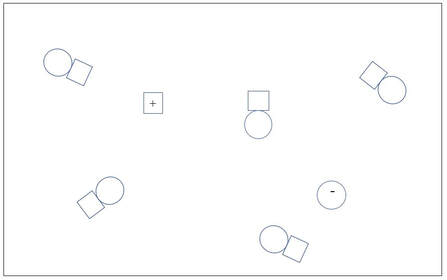
A
|
B
|
2. This questions involves comparison of the three solutions A, B, and C, listed here:
A 0.20 M aqueous sucrose solution
B 0.05 M aqueous sucrose solution
C 0.01 M aqueous sucrose solution
(a) Which one of these solutions is the strongest? Which is the weakest?
(b) Make some valid statements about the relative concentrations of the solutions A, B, and C.
3. Consider the following three aqueous solutions of hypothetical weak electrolytes HX, HY, and HZ.
A. 0.10 M solution of HA. 11% of the HX molecules are ionised, and 89% remain un-ionised.
B. 0.10 M solution of HB. 4% of the HY molecules are ionised, and 96% remain un-ionised.
C. 0.10 M solution of HC. 0.5% of the HZ molecules are ionised, and 99.5 % remain un-ionised.
(a) Make some valid statements about the relative strengths of the solutes HX, HY, and HZ.
(b) Why would your ability to answer question (a) be more uncertain if all of the solutions were not 0.10 M?
A. 0.10 M solution of HA. 11% of the HX molecules are ionised, and 89% remain un-ionised.
B. 0.10 M solution of HB. 4% of the HY molecules are ionised, and 96% remain un-ionised.
C. 0.10 M solution of HC. 0.5% of the HZ molecules are ionised, and 99.5 % remain un-ionised.
(a) Make some valid statements about the relative strengths of the solutes HX, HY, and HZ.
(b) Why would your ability to answer question (a) be more uncertain if all of the solutions were not 0.10 M?
4. Comment on what this person said: “I’m using quite a strong acetic acid solution – it’s 5.0 M. I think that I should use a weaker solution – like 0.01 M”.
5. Consider the following solutions:
Insert appropriate words in the spaces in the following sentences related to the two aqueous solutions A and B:
A: 0.01 M hydrochloric acid (HCl) solution
B: 0.08 M acetic acid (CH3COOH) solution.
C: 0.05 M sucrose (C12H22O11) solution
Insert appropriate terms in the spaces of the text below. Choose from the following words and phrases:
Solution A is more ……...…..…… (that is, less ……........……) than solution B. Solution B is ………......….. than solution C. The solute in solution A is a ………....…...………. electrolyte than the solute in solution B. In fact, because all of the HCl molecules ionise in solution, hydrochloric acid is a …………..…………. . The solute in solution B is a ……....…..…….. because only some of the molecules ……..........… in solution, but it is a ……..…........…… than the solute in solution C. In solution C, none of the sucrose molecules ionise, so sucrose is called a ….......…....…. .
Insert appropriate words in the spaces in the following sentences related to the two aqueous solutions A and B:
A: 0.01 M hydrochloric acid (HCl) solution
B: 0.08 M acetic acid (CH3COOH) solution.
C: 0.05 M sucrose (C12H22O11) solution
Insert appropriate terms in the spaces of the text below. Choose from the following words and phrases:
- concentrated
- more concentrated
- less concentrated
- dilute
- more dilute
- less dilute
- strong
- stronger
- weak
- weaker
- electrolyte
- strong electrolyte
- weak electrolyte
- non-electrolyte.
Solution A is more ……...…..…… (that is, less ……........……) than solution B. Solution B is ………......….. than solution C. The solute in solution A is a ………....…...………. electrolyte than the solute in solution B. In fact, because all of the HCl molecules ionise in solution, hydrochloric acid is a …………..…………. . The solute in solution B is a ……....…..…….. because only some of the molecules ……..........… in solution, but it is a ……..…........…… than the solute in solution C. In solution C, none of the sucrose molecules ionise, so sucrose is called a ….......…....…. .
6. Relational (or Venn) diagrams show the interrelationships among sets of objects or ideas. For example, a relational diagram can portray the relationships such as the following:
(a) Draw a relational (Venn) diagram that shows the interrelationships between the following concepts:
You might, for example, begin with trying to portray diagrammatically that every aqueous solution is either (i) a solutions of an electrolyte, or (ii) a solution of a non-electrolyte.
(b) Put each of the following solutions in the appropriate area of the diagram. That is, consider in which area of the diagram each solution “belongs”.
Remember that there is no single concentration that is a sharp dividing line between solutions that are classified as “concentrated” or “dilute”. Nevertheless, these terms are often used. Make practical decisions.
- In one set (category) of dogs (A), all are black. But not all dogs are black.
- In another set (B), all the dogs have short tails
- In another set (C), all the dogs have long hair
- A subset of the dogs in set A and a subset of those in set B have the common properties of being black with short tails
- A subset of the dogs in set A and a subset of those in set C have the common properties of being black with long hair.
- A subset of the dogs in set B and a subset of those in set C have the common properties of having short tails and long hair.
- A subset of the three subsets have the common properties of blackness, short tails and long hair.
(a) Draw a relational (Venn) diagram that shows the interrelationships between the following concepts:
- Solutions of an electrolyte
- Solutions of a strong electrolyte
- Solutions of a weak electrolyte
- Solutions of a non-electrolyte
- Concentrated solutions
- Dilute solutions
You might, for example, begin with trying to portray diagrammatically that every aqueous solution is either (i) a solutions of an electrolyte, or (ii) a solution of a non-electrolyte.
(b) Put each of the following solutions in the appropriate area of the diagram. That is, consider in which area of the diagram each solution “belongs”.
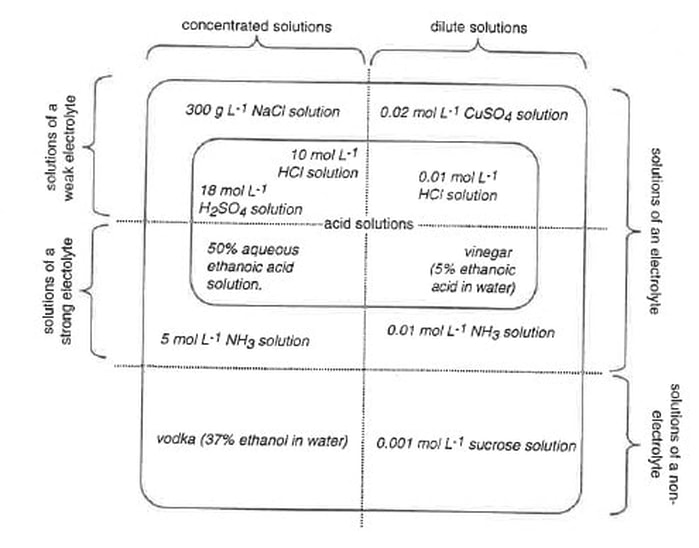
- vodka (37% ethanol in water)
- 300 g/L NaCl solution
- 50% aqueous ethanoic acid solution
- M sucrose solution
- M copper sulfate (CuSO4) solution
- 10 M HCl solution
- M ammonia (NH3) solution
- 18 M sulfuric acid (H2SO4) solution
- M HCl solution
- vinegar (5% ethanoic acid in water)
- 5 M ammonia (NH3) solution
Remember that there is no single concentration that is a sharp dividing line between solutions that are classified as “concentrated” or “dilute”. Nevertheless, these terms are often used. Make practical decisions.
Answers
1. A is a concentrated solution of a non-electrolyte solute.
B is a dilute solution of a weak electrolyte solute.
C is a dilute solution of a strong electrolyte solute.
D is a dilute solution of a non-electrolyte solute.
E is a concentrated solution of a weak electrolyte solute.
F is a A concentrated solution of a strong electrolyte solute
B is a dilute solution of a weak electrolyte solute.
C is a dilute solution of a strong electrolyte solute.
D is a dilute solution of a non-electrolyte solute.
E is a concentrated solution of a weak electrolyte solute.
F is a A concentrated solution of a strong electrolyte solute
2.
(a) These are nonsense questions. Words describing strength (such as strong, weak, stronger, weaker, …..) are relevant to properties of solutes in solution, not of the solutions.
(b) All of the following are valid statements about the solutions:
(a) These are nonsense questions. Words describing strength (such as strong, weak, stronger, weaker, …..) are relevant to properties of solutes in solution, not of the solutions.
(b) All of the following are valid statements about the solutions:
- A is a more concentrated solution (of sucrose) than both B and C.
- C is a more dilute (or, less concentrated) solution than both A and B.
- B is a more dilute solution than A, but is more concentrated than C
3.
(a) All of the following are valid statements about the solutes:
(b) The percentage of a weak electrolyte that ionises depends on the concentration of the solution. [The more dilute the solution, the more of the electrolyte ionises.] So the strengths of electrolytes can only be compared in solutions of the same concentration. (Although, in the example presented, the solutions would need to be of vastly different concentrations for the percentages ionised to be in a different order.]
(a) All of the following are valid statements about the solutes:
- HX is a stronger (weak) electrolyte than either of HY or HZ.
- HZ is a weaker (weak) electrolyte than both HX and HY.
- HY is a weaker electrolyte than HX, but a stronger electrolyte than HZ.
(b) The percentage of a weak electrolyte that ionises depends on the concentration of the solution. [The more dilute the solution, the more of the electrolyte ionises.] So the strengths of electrolytes can only be compared in solutions of the same concentration. (Although, in the example presented, the solutions would need to be of vastly different concentrations for the percentages ionised to be in a different order.]
4. Nonsense. Misuse of terms.
5. Solution A is more dilute … (that is less .. concentrated …) than solution B. Solution B is … more concentrated .. than solution C. The solute in solution A is a ... stronger .. electrolyte than the solute in solution B. In fact, because all of the HCl molecules ionize in solution, hydrochloric acid is a …. strong electrolyte …. . The solute in solution B is a …. weak electrolyte ... because only some of the molecules ... ionise … . in solution, but it is a … stronger electrolyte … than the solute in solution C. In solution C, none of the sucrose molecules ionise, so sucrose is called a … non-electrolyte .. .
6. There are many possible ways to represent the relationships among these categories of solution. One way is shown. The various solutions have been placed in their appropriate areas.
There is usually more than one way of diagrammatically representing relationships. No matter how the relational diagram is constructed, it should show:
- Of all the aqueous solutions of substances, some are solutions of non-electrolytes, and some are solutions of electrolytes.
- Of the aqueous solutions of electrolytes, some are solutions of strong electrolytes, and some are solutions of weak electrolytes.
- Regardless of whether the solute is a strong electrolyte, a weak electrolyte, or a non-electrolyte, solutions may be either concentrated or dilute.
Finding your way around .....
You can browse or search the Aha! Learning chemistry website in the following ways:
You can browse or search the Aha! Learning chemistry website in the following ways:
- Use the drop-down menus from the buttons at the top of each page to browse the modules chapter-by-chapter.
- Click to go to the TABLE OF CONTENTS (also from the NAVIGATION button) to see all available chapters and modules in numbered sequence.
- Click to go to the ALPHABETICAL INDEX. (also from the NAVIGATION button).
- Enter a word or phrase in the Search box at the top of each page.
1 Comment